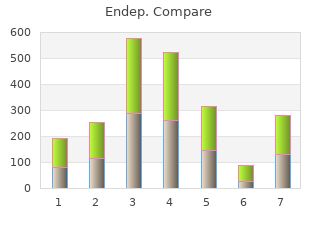
Endep
By M. Vak. Clayton College of Natural Health.
The commitment to voluntary male circumcision has grown to reach 6 buy endep 75 mg overnight delivery. A new component is the $210 million partnership with Gates and Nike for engaging adolescent girls and young women called DREAMS buy 25mg endep overnight delivery. They speak on prevention, vis-a-vis most at-risk populations, risk reduction, STI screening and treatment and comprehensive services for drug users, criminalization and stigma. They report on Operational Plans, including Training for Health Care Workers, by country and by region. The Global Fund The Global Fund to Fight AIDS, Tuberculosis and Malaria (GFATM) is an interna- tional financing institution that invests the world’s money to save lives. It supports large-scale prevention, treatment and care programs against the three diseases. Fiscal strategies were reviewed and redesigned in 2013 for accounting and forecast- ing. Risk management was updated in 2014 in finance, procurement and supply chain management. Total new grants for 2014 fell by close to 25%, to $2. There is almost ¾ of another billion USD signed but not committed to. From their 2014 Press Statement on Results, “Through the (new) funding model, the Global Fund is pursuing a differentiated approach to investing. It is weighing eco- nomic scenarios against epidemiological intelligence that points to diseases, espe- cially HIV and tuberculosis, becoming less generalized and more concentrated in certain locations and in key populations within a country. While certain middle- income countries and regions are making remarkable progress, others are falling back. Achieving control over these diseases calls for a diversified and differentiated approach, aligned with the Post-2015 development agenda. Ireland and Italy are in the “promised but not yet paid” column. UNAIDS UNAIDS provides technical support to countries, assisting them with expertise and planning for national AIDS programs, to help ‘make the money work’ for the people on the ground. UNAIDS tracks, evaluates and projects the financial resource require- ments at global, regional and country levels to generate reliable and timely infor- mation on the epidemic and the response. Based on these evaluations, UNAIDS pro- duces guidelines and progress reports. Much of the international data we juggle is set and approved by UNAIDS. They set out plans for “Getting to Zero” and other platitude-ridden slogans and programs. They are making a good effort on tackling major social issues like homophobia, financial sustainability and gender equality. The UN adopted a Political Declaration on HIV/AIDS in which member states agreed to increase investments for HIV to between $22–24 billion by 2015. A concerted effort by all countries is needed to meet the targets (slightly under $22B has been reached up until June 2015). Another promising approach would be to expand innovative mechanisms like indirect taxation (airline tickets, mobile phone usage, exchange rate transactions) to support global health initiatives, includ- ing HIV. The larger international community must continue to support and strengthen existing financial mechanisms, including the Global Fund and relevant UN organizations. The 15x15 program is a UNAIDS-sponsored program, as is the 90/90/90 idea, mentioned above and further on. The Bill and Melinda Gates Foundation The largest private philanthropic organization is located in Seattle, US, “focusing on improving people’s health and giving them the chance” to emerge from “hunger and extreme poverty. Much of these moneys are for non-AIDS-specific works, including development (reducing poverty and hunger). In health (58% of the total spending), they fight and prevent enteric and diarrheal diseases, malaria, pneumo- nia, TB, neglected and infectious diseases, working on integrated heath solutions, improving delivery of existing tools and supporting research and development in 6. Drugs available from whom and where FDA’s qualification of generics Generic drugs are important options that allow greater access to health care. They have the same high quality, strength, purity and stability as brand-name drugs. Generic manufacturing, packaging, and testing sites must pass the same quality stan- dards as those of brand name drugs. As of 2 July 2015, FDA had approved 187 generic drugs for use in the PEPFAR program that are approved in as short a time as six weeks. While quality, strength, purity and stability are guaranteed, adminis- tration, delivery and correct use is another issue. For example, the latest generic approved was another version of nevirapine.
It is recommended affecting the small airways that results in new fixed airflow that pulmonary function tests and chest x-rays are performed upon obstruction order endep 25mg on line. BOS occurs within the first 2 years after HCT endep 50 mg without prescription, but entry into long-term follow-up for at-risk patients and should be may develop as late as 4-5 years after transplantation. The repeated as clinically indicated in symptomatic patients and in those prevalence of BOS is 5. Influenza and and 14% among those who develop chronic GVHD. Patients usually present 4 health conditions encountered after HCT ; a brief description of with nonspecific symptoms, including shortness of breath, dry some of them follows. Unfortunately, once these symptoms develop, the degree of airflow obstruction is usually already significant and irreversible. Another common manifesta- Thyroid tion of BOS is the development of air-trapping, which can be Thyroid abnormalities include subclinical and overt hypothyroid- appreciated by high-resolution CT scans (persistent lucency of ism. Conversely, overt hypothyroidism is characterized by image that carries a sensitivity of 74%–91% and a specificity of low T4 levels accompanied with elevated TSH. Criteria used to make a clinical diagnosis of compensated hypothyroidism ranges from 25% to 30%, with a bronchiolitis obliterans include: (1) FEV1/FVC 0. The cumulative incidence of overt 75% of predicted value, (2) evidence of air trapping or small hypothyroidism ranges from 3. Hypothyroidism is related to radiation to the thyroid gland (3) absence of respiratory infection. Use of the airflow obstruction classification may permit study of early intervention Osteopenia/osteoporosis strategies; a recent consensus conference proposes inhaled 20-23 18 HCT recipients are at risk for osteopenia and osteoporosis. The corticosteroids and or inhaled bronchodilators for such cases. Patients with COP are more likely to incidence of osteoporosis approaches 20% at 2 years; the most have GVHD. It significant loss in bone mineral density occurs during the first 6 presents as an interstitial pneumonia and usually occurs within the months after HCT. Bone mineral loss increases the risk of fractures first 12 months after HCT. The clinical presentation in patients is in the HCT population just as it does in the general population; acute, with the sudden onset of a dry cough, dyspnea, and fever. Males with chronic GVHD and those exposed to secretion. The probability of developing gonadal failure increases calcineurin inhibitors are at increased risk of osteonecrosis. Recovery of spermatogenesis seems to occur more frequently in patients receiv- Recommendations for screening, early detection, and ing lower doses of cyclophosphamide (120 mg/kg) than in those prevention treated with higher doses (200 mg/kg). Male survivors have a A careful history and a thorough physical examination are the considerable chance of recovering sperm production even when cornerstone of screening. Early detection could result in the treated with TBI provided that they are 25 years of age at HCT utilization of surgical measures such as cortical decompression, thus and do not develop chronic GVHD. An important and potentially devastating complication of HCT is Although 50% of prepubertal girls exposed to fractionated TBI the occurrence of subsequent malignant neoplasms (SMNs). These include age at HCT, pre-HCT undergoing HCT develop ovarian failure, probably as a result of exposure to chemotherapy and radiation, exposure to TBI as part decreased reserve of primordial follicles. Irreversibility of ovar- of conditioning, infection with oncogenic viruses (EBV, HBV, ian function after HCT in most patients highlights the need for 5,30-32 and HCV), and prolonged immunosuppression after HCT. It timely hormonal replacement to prevent osteoporosis and other is conventional practice to classify SMNs into 3 distinct groups complications. Permanent gonadal damage and infertility are known toxicities of 27,28 Hodgkin lymphoma preparative regimens used for patients undergoing HCT. These cases differ from the EBV posttransplantation potential to influence quality of life (QOL); in fact, some survivors lymphoproliferative disorder by the absence of risk factors com- have reported that loss of fertility was as painful as confronting monly associated with that disorder, by a later onset ( 2. The high prevalence of infertility and the associate distress and by a relatively good prognosis. Although prevention radiation (both pre-HCT exposure and TBI) are associated with an Thyroid. Screening for thyroid dysfunction relies on a good history increased risk of solid tumors, several other factors (host and and physical examination, as well as annual thyroid function tests clinical) increase the risk of specific solid tumors. Survivors with abnormalities should be infection with oncogenic viruses (HBV, HCV: hepatocellular referred to an endocrinologist for hormone replacement. Males: Screening for hypogonadism should busulfan in smokers (lung cancer). The association between solid include an age-appropriate history and Tanner staging with attention tumor radiation therapy used to treat the primary cancer is typically to issues related to libido, impotence, or fertility. Measurement of associated with a long latency and the risk is high among those serum luteinizing hormone, follicle-stimulating hormone, and testos- exposed to irradiation at a young age. Therefore, among patients terone levels has been recommended as a baseline at age 11 years exposed to radiation at 30 years of age, the risk is 9-fold that of the and in boys whose puberty appears to be delayed. Abnormalities in general population, whereas for those 30 years, it approaches that testicular function should prompt a consultation with the endocrinolo- of the general population.
Quick-relief medications for asthma Page 89 of 113 Final Report Update 1 Drug Effectiveness Review Project Citation Exclusion Code FitzGerald JM buy endep 25 mg visa, Chapman KR purchase endep 25 mg mastercard, Della Cioppa G, et al. SHORT bronchoprotection, bronchodilatation, and symptom control during regular formoterol use in asthma of moderate or greater severity. Salmeterol in nocturnal 6 asthma: a double blind, placebo controlled trial of a long acting inhaled beta 2 agonist. Broncho-and cardioselective beta-receptor active drugs in 4 the treatment of asthmatic patients. Clinical studies of effects and side effects of terbutaline, practolol and metoprolol. Clinical comparison of inhaled terbutaline and orciprenaline 4 in asthmatic patients. Experiences of longterm treatment of asthma with 6-DESIGN terbutaline. A study on frequency of side effects and therapeutic effect. Terbutaline in COPD 6-POWDER comparison between Turbuhaler (R) and chlorofluorocarbon (CFC) inhaler. A study with cumulative doses of formoterol 6-LONG VS. Trial of new bronchodilator, terbutaline, in asthma. Risk of severe asthma episodes 5 predicted from fluctuation analysis of airway function. Comparison of a new multidose powder 6-POWDER inhaler with a pressurized aerosol in children with asthma. The effect of metaproterenol 6 in chronic asthmatic children receiving therapeutic doses of theophylline. The severity of asthma in relation to beta agonist 6-DESIGN prescribing. A dose-ranging trial of nebulized 5 (R)-albuterol (Levalbuterol) with racemic albuterol in pediatric patients with asthma. Comparative study of a new beta- 6 adrenergic stimulant in asthma: salbutamol. Gent PN, Hughes DT, Nisbet IG, Pearson SB, Sturgeon JG. Double- 5 blind comparative trial of two metered bronchodilator aerosols. Clinical comparability of 6-POWDER albuterol delivered by the breath-actuated inhaler (Spiros) and albuterol by MDI in patients with asthma. Bronchodilator effect of a new 3 oral beta adrenoreceptor stimulant, Th1165a. Quick-relief medications for asthma Page 90 of 113 Final Report Update 1 Drug Effectiveness Review Project Citation Exclusion Code Gibson GJ, Greenacre JK, Konig P, Conolly ME, Pride NB. Use of 6 exercise challenge to investigate possible tolerance to beta- adrenoceptor stimulation in asthma. Gilmartin JJ, Veale D, Murray A, Adams PC, Gibson GJ. Cardiac effects 6 of salbutamol given by air driven nebuliser at home. The salmeterol multicenter asthma research trial 5 (SMART). The use of Fenoterol (Berotec) respirator solution 0. Terbutaline: an effective bronchodilator by inhalation. SHORT pulmonary effects of high-dose formoterol in COPD: a comparison with salbutamol. SHORT of inhaled albuterol and two formulations of salmeterol on airway reactivity in asthmatic subjects. SHORT inhaled formoterol in children with asthma: a double-blind cross-over study versus salbutamol. Bronchodilator effects of 6 clemastine, ipratropium, bromide, and salbutamol in preschool children with asthma. SHORT Turbuhaler gave better protection than terbutaline against repeated exercise challenge for up to 12 hours in children and adolescents. Effects of an anticholinergic bronchodilator on 6 arterial blood gases of hypoxemic patients with chronic obstructive pulmonary disease. Grossman J, Geoffroy P, Hill MR, Vaughan LM, Nichols K.
The use of sodium valproate buy cheap endep 10mg line, carbamazepine and oxcarbazepine in patients with affective disorders generic endep 50 mg. Erzurumlu A, Dursun H, Gunduz S, Kalyon TA, Apracioglu O. The management of chronic pain at spinal cord injured patients. The comparison of effectiveness amitryptiline and carbamazepine combination and 3 electroacupuncture application. Antiepileptic drugs Page 112 of 117 Final Report Update 2 Drug Effectiveness Review Project Excluded studies Codes Faught E, Matsuo FU, Schachter S, Messenheimer J, Womble GP. Long- term tolerability of lamotrigine: data from a 6-year continuation study. A randomized open-label 6 month acute and maintenance trial of lamotrigine vs. An open prospective study of zonisamide in acute bipolar depression. Prophylactic sodium valproate therapy in patients with drug-resistant migraine. Methods & Findings in Experimental & Clinical 2 Pharmacology. Safety of carbamazepine extended-release capsules in bipolar disorder polypharmacy. Annals of Clinical Psychiatry Vol 18(Suppl1) May 5 2006, 19-22. Outcomes and length of treatment with carbamazepine extended-release capsules in bipolar disorder. Carbamazepine extended-release capsules use in bipolar disorder: Efficacy and safety in adult patients. Efficacy and Safety of Lamotrigine for Adults with Bipolar Disorder in a Private Practice Setting. Suicidal ideation and pharmacotherapy among STEP-BD patients. Psychiatric Services Vol 56(12) 5 Dec 2005, 1534-1540. Six-month prospective life charting of mood symptoms with lamotrigine monotherapy versus placebo in 2 rapid cycling bipolar disorder. Effect of divalproex on metabolic parameters is dose related in migraine prophylaxis. Oxcarbazepine (Trileptal) in the treatment of bipolar disorders: a review of efficacy and tolerability. Antiepileptic drugs Page 113 of 117 Final Report Update 2 Drug Effectiveness Review Project Excluded studies Codes Holmes LB, Smith CR, Hernandez-Diaz S. Pregnancy registries: larger sample sizes essential. Birth defects 5 research part A: Clinical and Molecular Teratology. A randomized, double-blind, multicenter, placebo- controlled 12-week study of the safety and efficacy of topiramate in patients 5 with acute manic or mixed episodes of bipolar I disorder with an optional open-label extension. Khoromi S, Patsalides A, Parada S, Salehi V, Meegan JM, Max MB. Weight change in the acute treatment of bipolar I disorder: a naturalistic observational study of psychiatric inpatients. Vigabatrin retinopathy in an Irish 3 cohort: lack of correlation with dose. Skin findings related to chronic usage of anti-epileptic drugs. Le Fauve CE, Litten RZ, Randall CL, Moak DH, Salloum IM, Green AI. Pharmacological treatment of alcohol abuse/dependence with psychiatric 5 comorbidity. Lenzi A, Lazzerini F, Grossi E, Massimetti G, Placidi GF. Use of Carbamazepine in acute psychosis: a controlled study. Epidemiological study of severe cutaneous adverse drug reactions in a city district of China. Valproate or olanzapine add-on to lithium: an 8-week, randomized, open-label study in Italian patients 3 with a manic relapse. A comparison study of the efficacy and tolerability between Depakote ER and Depakote in the acute treatment of mania and mixed 5 mania. Does gabapentin have an analgesic effect on background, movement and referred pain? A randomised, double-blind, placebo 4 controlled study.
N=69 Statins Page 159 of 395 Final Report Update 5 Drug Effectiveness Review Project Evidence Table 1 endep 75 mg fast delivery. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Clinical Trial Results (mean changes in lipoprotein levels) Harms/Comments Paoletti et al buy endep 25 mg low price. No serious AEs considered by the investigator to be 502 patients randomized rosuva 10mg: 49% (p<0. Trigs reduction from baseline at 12 weeks: 4), abdominal pain (2 vs. No parva: 13% clinically significant ALT or CK elevations. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Clinical Trial Funding Source Paoletti et al. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Inclusion Criteria/ Patient Clinical Trial Population Exclusion criteria Intervention Rawlings, 2009 Men with stale atherosclerosis and Unstable angina or revascularization within 3 months of study Atorvastatin 40 mg vs rosuvastatin 10 mg Multicenter (2 cardiology fasting LDL-C levels >=100 mg/dL enrollment, malignancy, chronic inflammatory disease, acute for 4 weeks clinics), double-blind off statin therapy. Presence of infection, history of myositis/myopathy, liver transaminases >2 times atherosclerosis determined by ULN, creatine phosphokinase greater than the ULN, and reluctance to >=50% stenosis in at least one discontinue statin therapy. Mean baseline LDL-C: 141 (SD 6) mg/dl N=30 Schneck et al, 2003 Men and women age 18 and older Pregnant or lactating women or women of childbearing potential not Atorva 10, 20, 40, or 80 mg qd or R, DB, MC with hypercholesterolemia and using a reliable form of contraception, as well as patients with a rosuvastatin 5, 10, 20, 40, or 80 mg qd for without active arterial disease history of heterozygous 6 weeks. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Clinical Trial Results (mean changes in lipoprotein levels) Harms/Comments Rawlings, 2009 Percent change from baseline, atorvastatin vs rosuvastatin: Not reported Multicenter (2 cardiology LDL-C: -45. Withdrawals due to adverse events infrequent (1 patient each in 374 patients randomized 61. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Clinical Trial Funding Source Rawlings, 2009 NIH and Foundations Multicenter (2 cardiology clinics), double-blind Schneck et al, 2003 Supported by R, DB, MC AstraZeneca Pharmaceuticals 374 patients randomized (n=165 aorta, 209 rosuva) 6 weeks Statins Page 164 of 395 Final Report Update 5 Drug Effectiveness Review Project Evidence Table 1. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Inclusion Criteria/ Patient Clinical Trial Population Exclusion criteria Intervention Schuster et al. Patients aged >=18 years, with Pregnant and lactating women, women not using reliable 6 week dietary lead-in phase, then 2004 CHD or other atherosclerotic contraception, patients with a history of homozygous familial randomization to 5 arm trial system R,OL,MC,ITT disease, type 2 diabetes, a CHD hypercholesterolemia or known type III hyperlipoproteinemia, with (drug a for 8 weeks then drug b or c for risk >20% over 10 years, with LDL- active arterial disease (e. Statins Page 165 of 395 Final Report Update 5 Drug Effectiveness Review Project Evidence Table 1. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Clinical Trial Results (mean changes in lipoprotein levels) Harms/Comments Schuster et al. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Clinical Trial Funding Source Schuster et al. Sponsored by Astra 2004 Zeneca R,OL,MC,ITT 5-arm trial that included statin switching (to rosuvastatin) at 8 weeks 3140 patients randomized 16 weeks of treatment Statins Page 167 of 395 Final Report Update 5 Drug Effectiveness Review Project Evidence Table 1. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Inclusion Criteria/ Patient Clinical Trial Population Exclusion criteria Intervention Schwartz et al, 2004 Patients aged >18 years, with LDL- Pregnant women, patients currently taking concomitant drugs known After a 6 week dietary lead-in, treatment C levels >=160 and< 250 mg/dL, to affect the lipid profile or to present a potential safety concern, a for the first 12 weeks: R, DB, MC and trig levels <=400 mg/dL, and history of active arterial disease (e. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Clinical Trial Results (mean changes in lipoprotein levels) Harms/Comments Schwartz et al, 2004 Efficacy analysis for 382 patients: "Although adverse events were frequently reported in these high-risk patients, % LDL-C change from baseline they were generally mild and not attributed to trial medication. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Clinical Trial Funding Source Schwartz et al, 2004 Sponsored by Astra Zeneca R, DB, MC 382 patients randomized 24 week treatment period Statins Page 170 of 395 Final Report Update 5 Drug Effectiveness Review Project Evidence Table 1. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Inclusion Criteria/ Patient Clinical Trial Population Exclusion criteria Intervention Stalenhoef et al. Also >3X ULN; and use of prohibited concomitant medications. Statins Page 171 of 395 Final Report Update 5 Drug Effectiveness Review Project Evidence Table 1. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Clinical Trial Results (mean changes in lipoprotein levels) Harms/Comments Stalenhoef et al. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Clinical Trial Funding Source Stalenhoef et al. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Inclusion Criteria/ Patient Clinical Trial Population Exclusion criteria Intervention Strandberg et al, 2004 Men and women >=18 years with A history of serious adverse events or hypersensitivity to an hMG-CoA rosuv 10 mg/d LDL-c level >135 mg/dL for statin- reductase inhibitor other than the study drugs; active hepatic disease; atorv 10 mg PO OD R (2:1), OL, MC, 2-arm naïve patients or >120 mg/dL in homozygous or heterozygous familial hypercholesterolemia (FH); study, ITT patients using the starting dose of unstable angina; elevated serum creatinine concentration (>220 optional extension period for rosuv pts who another lipid-lowering drug. Statins Page 174 of 395 Final Report Update 5 Drug Effectiveness Review Project Evidence Table 1. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Clinical Trial Results (mean changes in lipoprotein levels) Harms/Comments Strandberg et al, 2004 Efficacy analysis for 911 patients (rosuv 10mg/d, n= 627; atorv 10mg/d, n= Patients experiencing any AE (estimated from graph): 284) Rosuv ~38% (n=261) R (2:1), OL, MC, 2-arm Atorv ~37% (n=125). Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Clinical Trial Funding Source Strandberg et al, 2004 Supported by a grant from AstraZeneca R (2:1), OL, MC, 2-arm study, ITT 1024 patients randomized (n=686 to rosuv 10 mg/d, n=338 to atorv 10 mg/d) 12 weeks Statins Page 176 of 395 Final Report Update 5 Drug Effectiveness Review Project Evidence Table 1. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Inclusion Criteria/ Patient Clinical Trial Population Exclusion criteria Intervention Wolffenbuttel et al. Men and women with type 2 use of lipid-lowering drugs after visit 1, or a history of serious or After a 6-week dietary lead-in, treatment 2005 diabetes who had received hypersensitivity reactions to statins. Concomitant treatment with erythromycin, clarithromycin, azole antifungal agents, cyclosporin, antiviral agents, phenytoin, carbamazepine, phenobarbital, or nefazodone. Statins Page 177 of 395 Final Report Update 5 Drug Effectiveness Review Project Evidence Table 1. Trials comparing LDL-c lowering/HDL-c raising abilities of 2 or more statins Clinical Trial Results (mean changes in lipoprotein levels) Harms/Comments Wolffenbuttel et al.
CY-BOCS purchase endep 50 mg with amex, Children’s Yale-Brown Obsessive Compulsive Scale The focus of the 2 aripiprazole trials was the treatment of irritability order endep 25 mg without a prescription, as assessed by the ABC Irritability subscale. This scale includes items such as “injures self,” “physical violence to self,” “aggressive to other children and adults,” “irritable,” “temper outbursts,” “depressed 513, 514 mood,” “mood changes,” and “yells” or “screams” inappropriately. In both studies, children and adolescents taking aripiprazole showed greater improvement in irritability at 8- week follow-up than those randomized to placebo. Additional analyses of these trials are 517, 518 available in conference posters. A poor-quality placebo-controlled trial of olanzapine in 11 children and adolescents with pervasive developmental disorders reported that 50% of subjects improved with olanzapine compared with 20% with placebo on the primary outcome, the Clinical Global Impression- 515 Improvement (CGI-I) scale (P value not reported). There were no significant differences between treatment groups on other measures of irritability and aggression. Risperidone was studied in 5 fair-quality placebo-controlled trials that enrolled children with autistic disorder, Asperger’s disorder, or pervasive developmental disorder not otherwise 508-512 510, 511 specified. One of these enrolled preschool age children with autistic disorder or pervasive developmental disorder not otherwise 510 specified. When baseline motor development and language skills were controlled for, there was no difference between risperidone and placebo on the Childhood Autism Rating Scale at study endpoint. The other 6-month study enrolled 40 children with autistic disorder ages 2 to 9 511 years. At follow-up, children taking risperidone showed greater improvement on the Childhood Autism Rating Scale and the Children’s Global Assessment Scale (GAS). Parents reported no significant changes in restricted interests, emotional interaction, verbal communication, or speech. In 3 short-term trials, risperidone showed greater efficacy compared with placebo in 508, 509 512 improving symptoms or preventing relapse at 8 weeks. One of these studies, the RUPP Atypical antipsychotic drugs Page 128 of 230 Final Report Update 3 Drug Effectiveness Review Project Trial, included a 4-month open-label extension phase, followed by an additional 8-week placebo- controlled discontinuation phase. Fifty-one children completed the 4-month open-label treatment period; 5 were withdrawn because of loss of efficacy, 1 because of noncompliance with the protocol, 1 dropped out due to constipation, 1 withdrew consent, and 4 were lost to follow-up. There was a slight increase in mean irritability ratings over the extension phase, but mean scores were still reduced from pretreatment baseline levels and 82. The placebo-controlled discontinuation phase of this study included 38 of 101 children who had a positive response to risperidone after 4 507 months of open-label treatment. The trial was stopped after 32 patients completed the discontinuation phase, after review by a Data and Safety Monitoring Board found a significantly higher relapse rate in the placebo group: 62. The applicability of these results to children seen in general practice is severely limited because they represent a highly selected group (less than one-third of those who enrolled in the original 8-week trial) who responded well to risperidone and were able to comply with the protocol. No conclusions about comparative efficacy of the different atypical antipsychotics can be drawn from these placebo-controlled trials because the trials differed in their populations (age, diagnosis), durations, and outcome measures. Active-control trials There were 2 fair-quality, active-control trials of atypical antipsychotics compared with 516, 519 haloperidol in children or adolescents with autistic disorder. There was no difference between treatment groups on the CGI- 516 I scale at 6-week follow-up (P=0. There was a trend for greater improvement with olanzapine on the Clinical Global Impression-Severity (CGI-S) scale and the Conners Parent Rating Scale (CPRS), but the difference was not statistically significant. This open-label trial enrolled only 12 patients and was considered a pilot study. The trial comparing risperidone to haloperidol included a 12-week randomized treatment 519 520 phase followed by a 12-week open-label maintenance phase. At 12 weeks, there was a greater improvement from baseline with risperidone on the ABC (P=0. There was no difference between groups, however, on the CGI-I scale or the Ritvo-Freeman Real Life Rating Scale. Of the 30 children and adolescents who entered the 12- week treatment phase, 28 continued in the 12-week open-label maintenance phase. At 24 weeks, there was greater improvement from baseline with risperidone compared with haloperidol on the CGI-I scale (P=0. There was also a trend for greater improvement with risperidone on the ABC (P=0. There was no difference between groups on 4 of 5 subscales of the Ritvo-Freeman Real Life Rating Scale, with greater improvement on the language subscale only with risperidone (P=0. Observational studies 521-529 We identified 9 observational studies with efficacy outcomes in patients with autism, but none were comparative, and none reported functional outcomes. Atypical antipsychotic drugs Page 129 of 230 Final Report Update 3 Drug Effectiveness Review Project Disruptive behavior disorders Disruptive behavior disorders included the diagnoses of conduct disorder, oppositional defiant disorder, and disruptive behavior disorder not otherwise specified. There were no head-to-head or active-control trials in this population. Most were short-term efficacy trials of 6 to 10 weeks in duration. Two risperidone trials were 530, 532 conducted simultaneously using identical designs. Both of these used the Nisonger Conduct Problem subscale as the primary outcome measure.
Curative efficacy of ondansetron against nausea and emesis induced by anticancer drugs: A study versus 2 metoclopramide endep 10 mg on line. Antiemetics Page 102 of 136 Final Report Update 1 Drug Effectiveness Review Project Exclusion Excluded Studies code # Marty M purchase endep 25mg amex, Paillarse JM, the French Study G. Efficacy of ondansetron (ONC) and metoclopramide (MCP) as an intervention treatment in patients 2 experiencing emesis. Comparison of the 5-hydroxytryptamine3 (serotonin) antagonist ondansetron (GR 38032F) with high-dose 2 metoclopramide in the control of cisplatin-induced emesis. Mehta NH, Reed CM, Kuhlman C, Weinstein HJ, Parsons SK. Controlling conditioning-related emesis in children undergoing bone marrow 2 transplantation. Randomised double-blind comparison of ondansetron and droperidol to prevent postoperative nausea 2 and vomiting associated with patient-controlled analgesia. Prevention of chemotherapy- induced emesis with granisetron in children with malignant diseases. Ondansetron is not superior to moderate dose metoclopramide in the prevention of post-operative nausea 2 and vomiting after minor gynaecological surgery. The addition of antiemetics to the morphine solution in patient controlled analgesia syringes used by children 2 after an appendicectomy does not reduce the incidence of postoperative nausea and vomiting. Munstedt K, Milch W, Blauth-Eckmeyer E, Spanle A, Vahrson A, Reimer C. Prevention of cisplatinum-induced delayed emesis and nausea. Mustacchi G, Ceccherini R, Leita ML, Sandri P, Milani S, Carbonara T. The combination of Metoclopramide, Methylprednisolone and Ondansetron 2 against antiblastic-delayed emesis: A randomised phase II study. Mustacchi G, Ceccherini R, Milani S, Sandri P, Leita ML. Ondansetron (O), metoclopramide (M) and methylprednisolone (MP) p. Mylonakis N, Tsavaris N, Karabelis A, Stefis J, Kosmidis P. A randomized comparative study of antiemetic activity of Ondansetron (Ond) vs Tropisetron 2 (Tr) in patients receiving moderately emetogenic chemotherapy. Granisetron plus methylprednisolone versus granisetron alone in prevention of emesis associated with cisplatin- 2 containing chemotherapies. Antiemetics Page 103 of 136 Final Report Update 1 Drug Effectiveness Review Project Exclusion Excluded Studies code # Navari RM, Province WS, Perrine GM, Kilgore JR. Comparison of intermittent ondansetron versus continuous infusion metoclopramide used with standard 2 combination antiemetics in control of acute nausea induced by cisplatin chemotherapy. Nicolai N, Mangiarotti B, Salvioni R, Piva L, Faustini M, Pizzocaro G. Dexamethasone plus ondansetron versus dexamethasone plus alizapride in 2 the prevention of emesis induced by cisplatin-containing chemotherapies for urological cancers. Numbenjapon T, Mongkonsritragoon W, Prayoonwiwat W, Sriswasdi C, Leelasiri A. Ogihara M, Suzuki T, Yanagida T, Tsuruya Y, Ishibashi K, Yamaguchi O. Clinical assessment of granisetron and methyl-prednisolone as a 2 prophylactic antiemetic in cisplatin-induced delayed emesis. A randomized cross-over study of high-dose metoclopramide plus dexamethasone versus granisetron plus 2 dexamethasone in patients receiving chemotherapy with high-dose cisplatin. Efficacy and tolerability of granisetron with betamethasone, an antiemetic combination, in 2 gynecologic cancer patients receiving cisplatin. Current Therapeutic Research - Clinical and Experimental. Granisetron in the prevention of vomiting induced by conditioning for stem cell transplantation: A prospective 2 randomized study. Aprepitant in antiemetic combinations to prevent chemotherapy- 2 induced nausea and vomiting. Comparison of granisetron with granisetron plus droperidol combination prophylaxis in post- 2 operative nausea and vomiting after laparoscopic cholecystectomy. The effect of anaesthetic technique on postoperative nausea and vomiting after day-case gynaecological 2 laparoscopy. Antiemetics Page 104 of 136 Final Report Update 1 Drug Effectiveness Review Project Exclusion Excluded Studies code # Peixoto AJ, Peixoto Filho AJ, Leaes LF, Celich MF, Barros MAV. Efficacy of prophylactic droperidol, ondansetron or both in the prevention of postoperative nausea and vomiting in major gynaecological surgery.