Mildronate
By C. Owen. Saint Edwards University.
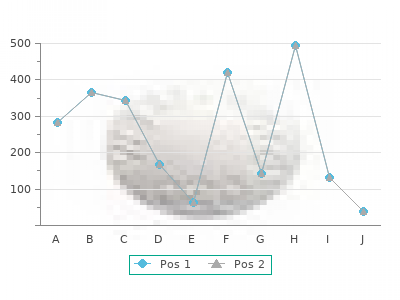
Acute toxicity study of aqueous extract of dried whole plant were evaluated in mice cheap 250 mg mildronate with visa. The aqueous extract was not toxic up to the maximum feasible dose level of 8g/kg body weight mildronate 250mg without prescription. The hypoglycaemic effect of aqueous extract (3g/kg body weight) was carried out on adrenaline-induced diabetic rabbits. It was found that aqueous extract (3g/kg body weight) which is 120 times human doses showed significant lowering the blood glucose levels at 1hr and 3hr respectively (p< 0. Khine Khine Lwin; Mu Mu Sein Myint; May Aye Than; Khin Tar Yar Myint; Win Win Maw; San San Myint; Myint Myint Khine; Nu Nu Win; Hla Phyo Lin. Six adrenaline-induced hyperglycaemic rabbits were used to study the hypoglycaemic effect. To compare the hypoglycaemic effects of this plant wiht that of the standard drug, hypoglycaemic effect of glibenclamide (4mg/kg) was also investigated. The phytochemical studies of the crude powder and 80% ethanolic exteact of this plant showed that both contained alkaloid, flavonoid, glycoside, steroid, saponin, tanin and amino acid. In acute toxicity atudy in mice, it was observed that the crude powder of rhizomes of this plant was not toxic up to the maximal feasible dose of (5g/kg). The results showed that the 80% ethanolic extract of the rhizomes of this plant at the dose level of (1. But, the crude powder of the rhizomes of this plant at the dose level of (3g/kg) showed no significant hypoglycaemic effect. It was observed that the hypoglycaemic effect of 80% ethanolic extract of this plant was inferior to that of the standard drug, glibenclamide. A clinical trial to determine the hypoglycemic potential of popular Myanmar medicinal plant Orthosiphon aristatus Bl. A significant blood sugar lowering effect was observed 1hr after administration of 175ml of plant decoction extracted from 25g leaves on glucose- loaded (75g glucose) model when compared to glucose loaded control group. There was no effect on liver function tests, kidney function tests blood urea and electrolyte, serum creatinine and serum cholesterol level. First group of 10 patients received 75g of glucose together with 175ml plant decoction at the same time (group A). A second group of 10 patients, 175ml of plant decoction was given first; 75g glucose load was given 2hrs later (group B). The effect of 500mg of glucophage together with 75g glucose was also determined on the same group of patients for positive control. Significant blood sugar lowering effects were observed in both group A and group B 3 hours after administration of plant decoction. There was statistical significant reduction of blood sugar level in both group A and group B patients when compared to the control group (p<0. There was more reduction of blood sugar level in both receiving See-cho-pin (group A and group B) when compared to patients receiving glucophage 500mg. Khin Chit; Ohnmar May Tin Hlaing; Phyu Phyu Aung; Tin Tin Aung; Win Win Myint; Khine Khine Lwin; Aye Than; Phyu Phyu Win; San San Win. A clinical trial to determine the hypoglycemic effect of Orthosiphon aristatus (Bl. Individual diet instruction as prescribed by dietitian of Nutrition Research Division was distributed to each patient. After the control study, the patient was given 8gm of dried leaf in 250ml boiled water for 30mins, 3 times per day for 28 days. The effect of gliclazide 80mg for a period of 28 days was also studied on the positive control group of six patients. There was a statistically significant reduction of blood sugar level in patients receiving See-cho-pin plain tea (p<0. A significant blood sugar lowering effect was also observed in patients receiving gliclazide (p<0. There was no significant difference in the blood sugar lowering effect among the group receiving gliclazide and the group receiving See-cho-pin plain tea after a complete wash out period. No significant side effect of See-cho-pin plain tea was observed clinically during the study. Hypoglycemic effect of “Paya-say”, prepared from traditional method, on rabbit model. The aim of this study is to determine acute toxicity and the hypoglycaemic effect of “Paya-say”, prepared from traditional method. The “Paya-say”, was not toxic up to the maximum feasible dose level of 53ml/kg body weight. It was found that “Paya-say”, 15ml/kg body weight showed not significantly lowered the blood glucose levels at 1hr, 2hr, 3hr and 4hr respectively.

After three months generic mildronate 500 mg with visa, pain and tenderness were significantly reduced in both the women with cyclic breast pain and those with noncyclic pain cheap mildronate 500 mg without a prescription. When larger numbers of women were studied, vitamin E did not fare so well, showing no significant effects either subjectively or objectively. This hypersensitivity can produce excessive amounts of secretions, distending the breast ducts and producing small cysts and later fibrosis (hardening of the tissue due to the deposition of fibrin, similar to the formation of scar tissue). Results from these studies indicate that although treatment with high doses of iodides was effective in about 70% of subjects, it was associated with a high rate of side effects (altered thyroid function in 4%, iodinism in 3%, and acne in 15%). We recommend that patients take iodine only under strict medical supervision, as taking too much iodine can lead to altered levels of thyroid hormone. In addition to iodine, there is research showing that thyroid hormone replacement therapy may result in clinical improvement. For more information on subclinical hypothyroidism, see the chapter “Hypothyroidism. The improvement in breast pain was greater in the chasteberry group (52%) compared with the placebo group (24%). Food Allergy • Significant improvement in symptoms and signs of a disease linked to food allergy while on an allergy-elimination diet • Positive test result from an acceptable food allergy test • Typical signs of allergy: Dark circles under the eyes (allergic shiners) Puffiness under the eyes Horizontal creases in the lower eyelid Chronic (noncyclic) fluid retention Chronic swollen glands A food allergy occurs when there is an adverse reaction to the ingestion of a food. The reaction may or may not be mediated (controlled and influenced) by the immune system. The reaction may be caused by a protein, a starch, or another food component, or by a contaminant found in the food (a coloring, a preservative, etc. A classic food allergy occurs when an ingested food molecule acts as an antigen—a substance that can be bound by an antibody. Antibodies are the protein molecules made by white blood cells that bind to foreign substances, in this case various components of foods. The food antigen is bound by antibodies known as IgE (immunoglobulin E) for immediate reactions and IgG and IgM for delayed reactions. The IgE antibodies are specialized immunoglobulins (proteins) that bind to specialized white blood cells known as mast cells and basophils. When the IgE and food antigen bind to a mast cell or basophil, the binding causes a release of histamines, substances that in turn cause swelling and inflammation. Other terms often used to refer to food allergy include food hypersensitivity, food anaphylaxis, food idiosyncrasy, food intolerance, pharmacological (drug-like) reaction to food, metabolic reaction to food, and food sensitivity. The recognition of food allergy was first recorded by the Greek physician Hippocrates, who observed that milk could cause gastric upset and hives. He wrote, “To many this has been the commencement of a serious disease when they have merely taken twice in a day the same food which they have been in the custom of taking once. Allergies have also been linked to numerous disorders of the central nervous system, including depression, anxiety, and chronic fatigue. The actual symptoms produced during an allergic response depend on the location of the immune system activation, the mediators of inflammation involved, and the sensitivity of the tissues to specific mediators. As is evident in the table opposite, food allergies have been linked to many common symptoms and health conditions. It is estimated that 6% of children and 4% of adults in America have IgE-mediated food allergies2 and that 20% of the population have altered their diet owing to adverse reactions to foods. The primary causes of the increased frequency of food allergy appear to be excessive regular consumption of a limited number of foods (often hidden as ingredients in commercially prepared foods) and the high level of preservatives, stabilizers, artificial colorings, and flavorings now added to foods. For example, foods can easily become contaminated following the use of pesticides in farming. Other possible reasons for the increased occurrence of food allergy include earlier weaning and earlier introduction of solid foods to infants; genetic manipulation of plants, resulting in food components with greater allergenic properties; and impaired digestion (especially lack of hydrochloric acid and/or pancreatic enzymes). Finally, incomplete digestion and excessive permeability of the intestinal lining significantly contribute to the risk of becoming allergic to foods. When both parents have allergies, there is a 67% chance that the children will also have allergies. When only one parent is allergic, the chance that a child will be prone to allergies is still high but drops from 67 to 33%. The theory is that individuals with a tendency to develop food allergies have abnormalities in the number and ratios of special white blood cells known as T lymphocytes or T cells. Specifically, these individuals have nearly 50% more helper T cells than nonallergic persons. Individuals prone to food allergies have a lower allergic set point because they have more helper T cells in circulation. Therefore, the level of insult required to trigger an allergic response is lowered.
The most significant outcome of the numerous cell marking and therapeutic trials appears to be a lack of observed toxicity due to gene transfer 500mg mildronate otc. However discount mildronate 250 mg amex, a recent clinical trial has reported one death due to the approved experimental protocol (see Chapter 13). Additionally, it has come to light that other deaths have occurred in gene therapy clinical trials. However, it is unclear whether these deaths are related to the experimental therapy. The majority of human gene therapy protocols involve cancer, and the most common viral vector in use is the retrovirus. Most cancer studies are gene-marking studies where a cell is marked with a gene to elucidate metatasis or recurrence. The limited clinical experience to date does not rule out long-term adverse effects from gene therapy protocols as noted in Chapter 13. Thus, the ability to bring recent laboratory-based advances to the bedside relies on the quantity and quality of the underlying science, the carefulness used in clinical protocol design and outcome measure, as well as a multidisciplinary approach to bridging basic science and medicine. Currently, numerous basic science issues need to be addressed in the development of human gene therapy protocols. Gene Transfer Gene transfer can be achieved by two methods: direct transfer (in vivo) or laboratory manipulation (ex vivo). Utilizing these methods, gene transfer should be administered to the patient without adverse side effects. Various gene transfer protocols (systems) are currently under development and should be tailored to the clinical condition. In principle, studies in yeast have indicated that the development of artificial chromosome vectors may allow for the maintenance of transferred genes and obviating the problems of random insertion of viral constructs. Gene Expression Once a gene is transferred into a tissue or cell, expression of that gene is necessary for successful gene therapy. Currently, however, persistent high levels of gene expression are not consistently achieved in gene therapy protocols. It is unclear whether these experimental data reflect unknown cellular mechanisms needed for therapeutic gene expression, a selective disadvantage of the use of stem cells expressing transferred genes, or the failure to include appropriate regulatory elements in current gene constructs. What is clear from current human studies is that protocols that produce high levels of gene expression in mice do not reproduce similar gene expressions in clinical studies. Long-term expression of transferred genes and high levels of gene product have been reported in murine studies. But a deficiency arises when comparable pro- tocols are employed in clinical studies. Studies have relied on molecular methods of detection of gene expression rather that direct protein assays. Thus, at the current stage the lack of expression of transferred genes compromises both the clinical benefit and scientific value of gene therapy. Gene Targeting Gene therapy approaches could be enhanced by directing gene transfer and expres- sion to specific cells or tissues (see Chapter 5). Using such an approach would reduce the need for gene targeting required with in vivo transfer techniques. However, current ex vivo techniques could be enhanced by using targeting techniques such as that used in liver-cell-directed gene therapy (see Chapter 7). The use of ligands that bind to surface receptors could augment gene incorporation into the cell. Disease Pathology The identification of a genetic mutation as a cause of disease pathology is an im- portant step in gene therapy. However, equally important is the elucidation of the biological mechanisms through which the mutated polypeptide molecule induces pathogenesis. Mutations may cause loss of function so that gene therapy replaces the mutated gene product sufficiently for effective therapy. However, somatic muta- tion may also be dominant negative in the biological mechanism. Here, the mutated protein inhibits a cellular metabolic pathway and a therapeutic approach would be to delete expression of the mutated protein. Therefore, a detailed understanding of the pathophysiology of the disease is required for designing gene therapy protocols. Both the genes in question need to be revealed as well as the cellular targets that could be utilized for therapy. For example, skin or muscle cells could be targeted for systemic diseases as opposed to liver cells. Regardless, the use of gene therapy to further understand disease pathophysiology could lead to the development of novel therapeutic approaches to disease remission. Animal Models of Disease As a correlate to the study of disease pathogenesis in the context of gene therapy, animal models of human disease provide the principles of disease pathogenesis (see Chapter 3).
These IvIg are directed primarily either against antigens present on the surface of the infecting microorganisms or towards factors released generic mildronate 250 mg visa, including en- dotoxin buy discount mildronate 500 mg online, peptidoglycans and lipoteichoic acid, when the organism is killed by antibiotics. The second approach is based on administering antibodies directed against speci¿c sepsis mediators or on neutralising their receptors on the cell surface. To be inactivated, a foreign substance must react with ¿xed or circulating receptors, which trigger the ¿nal response. This task is accomplished by two distinct but strictly co-operating systems [6, 7]. The number of receptors present on the surface of innate immune system cells is genetically determined and, albeit suf¿cient in number, cannot match the wide variability of microbial antigenic epitopes. Thus, a more Àexible system is required in order to face the myriad of agents and/or substances that come into contact with the host. This second mechanism, known as adaptive immunity due to its capability to cope with continuously changing antigens, involves antibodies, which are encoded by genes that are able to undergo somatic recombi- nation and hypermutation. The IgG class is considered the prototypical structure and consists of a Y-shaped molecule composed of two identical heavy (H) and light (L) peptide chains 20 Immunoglobulins in Sepsis 237 Table 20. Both H and L chains are divided into a variable (V) domain that reacts with the antigen and a constant (C) region that activates the various components of the innate immune system, triggering a response (for example, phagocytosis, antibody-mediated and cell-mediated cytotoxicity and complement-mediated lysis). The region connecting the two functional parts can undergo conformational changes to reshape the molecule accord- ing to antigen variability. Therefore, Ig can be considered biochemical transducers that are able to: • recognise invading micro-organisms and derived substances; • opsonise bacteria; • signal their presence directly or via the complement cascade to the cells of the innate immune system, which are ultimately responsible for their destruction; • neutralise bacteria-derived toxins [1, 7] (Table 20. Immunoglobulins are widely used as both therapeutic and diagnostic tools in many ¿elds of medicine. On the basis of their speci¿city, IvIg preparations can be grouped into monoclonal – containing a single class of Ig directed against a single epitope of those present upon a target molecule (e. The additional immunomodula- tory effects attributed to the latter class are due to naturally occurring autoantibodies and some nonimmune proteins present in the preparation [1]. Only post hoc analyses could identify, in some studies, small subsets of patients who bene¿ted from this approach [5]. Various clinical reasons have been proposed as explanation for these contradictory results, including the role of coexisting disease in determining the outcome, the choice of 28- or 56-day survival as study endpoints, and the appropriateness of con- comitant treatments [10]. Another possible reason may involve the treatment itself and the rationale behind monoclonal Ig therapy. As sepsis mediators are linked by multiple posi- tive and negative feedback loops, the blockade of only one of the substances responsible for the initial phase (i. Despite the disappointing results of trials studying septic patients, antibod- ies directed against some of these substances are currently used to treat disorders such as Crohn’s disease and rheumatoid arthritis, which, in contrast to sepsis, are characterised by a chronic and localised rather than acute and systemic inÀammatory reaction [12]. Polyclonal preparations contain variable amounts of Ig directed against a variety of Gram- negative and Gram-positive epitopes and bacteria-derived substances, including endotoxin. Several preparations containing predominantly IgG with only traces of other Ig are available (Polyglobin®, Bayer, Germany), whereas only one product contains elevated concentrations of IgM (in addition to IgG) and minor amounts of IgA (Pentaglobin®, Biotest, Germany) (eIg). Aside from Ig concentration, the various preparations also differ with regard to the sta- bilisers used [1]. Their popularity can be attributed to the wide- spread occurrence among critically ill patients of conditions associated with a downregula- tion of their immune capabilities, including postoperative status [14] and neoplasms [15]. Several investigators have studied the effects of prophylactic IvIg 20 Immunoglobulins in Sepsis 239 administration in different categories of patients prone to infections and sepsis, including premature infants and patients undergoing heart surgery. As far as the ¿rst category is concerned, a marginal reduction of early-onset neonatal sepsis has been demonstrated by a meta-analysis in premature newborns with a low birth weight [17]. However, this issue should continue to be considered as open, as other studies have failed to identify any sur- vival bene¿t in this group of patients [18]. Another study investigating the effects of eIg con¿rmed these results, demonstrating that the bene¿cial effect was limited to more seriously ill patients who developed severe post- operative sepsis [23]. The weakness of investigations concerning polyclonal IvIg use in sepsis – and therefore of the derived meta-analysis – lies in the relatively small number of patients studied and the heterogeneity of their underlying conditions [27]. A bene¿cial effect of polyclonal IvIg has also been demonstrated in less frequently encountered critically ill patients who have toxic shock syndrome secondary to severe streptococcal group A infections [28, 29]. With regard to the type of preparation of IvIg, different meta-analyses have demonstrated an increased survival rate in patients treated with eIg compared with preparations containing IgG alone [30, 31]. As the endotoxin molecule represents a target for IgM [14], this effect is particu- larly evident in patients who have Gram-negative infection [32]. A number of studies indicate that polyclonal IvIg administration is associated with ei- ther reduced morbidity or improved survival rate in different populations of patients with sepsis, severe sepsis and septic shock [33–36]. The improved survival rate is more marked in certain subsets of patients, such as those who have sepsis sustained by Gram-negative bacteria [2, 32]. However, it is important to stress that IvIg should be considered as an ad- junctive treatment that integrates with, but does not replace, appropriate antibiotic therapy, 240 G.