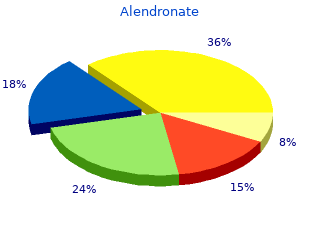
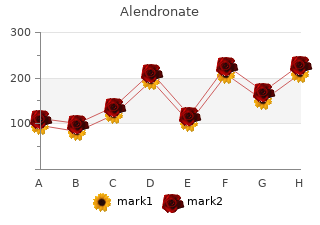
Alendronate
2019, New York Academy of Art, Murat's review: "Alendronate 70 mg, 35 mg. Purchase online Alendronate cheap no RX.".
After flowering discount alendronate 70 mg fast delivery, sessile maximum daily dose should not exceed the equivalent of 3 generic 70mg alendronate mastercard. Hagers Handbuch der Not to be Confused With: The herb should not be confused Pharmazeutischen Praxis. Other Names: Broom, Broomtops, Besom, Scoparium, Irish Nicolic R et aL, Acta Pharm Jugosl 26:257. Chemie, sparteine, including among others 11, 12-dehydrosparteine, Pharmakologie, 2. Tyramine acts indirectly on Scotch Broom the sympathetic nervous system as a vasoconstrictor and hypertensive. Sparteine acts negatively inotrop- (approximately 30 gm of the drug), lead to dizziness. Spasms are to be treated with Approved by Commission E: chlorpromazine or diazepam. No • Circulatory disorders deaths through poisonings with this drug have been proven • Hypertension beyond a doubt (though they certainly have been with Unproven Uses: The herb is used for functional heart and sparteine). Unproven Uses: The use of the pure drug cannot be recommended except as an inactive ingredient in teas. The drug is contraindicated in high blood pressure, A-V Storage: Carefully protect from light and moisture. To be used only administration of designated therapeutic dosages are not under the supervision of an expert qualified in the appropri- recorded. Preparatfon: To prepare an infusion, Pour 200 ml boiling Drug Interactions: Use of Scotch Broom herb with mono- water over 1 teaspoon of flowers and strain after 10 minutes. For pathologi- cal edema, 1 liter infusion per day is administered in 4 Pregnancy: The herb should not be used during pregnancy portions during meals for 1 month. Aufl, Bde 4-6 (Drogen): Springer Verlag Berlin, Heidelberg, New York, 1992- Madaus G, Lehrbuch der Biologischen Arzneimittel, Bde 1-3, 1994. Daunderer M, Kormann K, Giftpflanzen, Pflanzengifte, structure of the Cytisus scoparius seed lectin and a 4. Encyclopedia of Common Natural Ingredients Used Teuscher E, Lindequist U, Biogene Gifte - Biologie, Chemie, in Food, Drugs and Cosmetics, John Wiley & Sons Inc. Phytopharmaka und Madaus G, Lehrbuch der Biologischen Arzneimittel, Bde 1-3, pflanzliche Homoopathika, Fischer-Verlag, Stuttgart, Jena, New Nachdruck, Georg Olms Verlag Hildesheim 1979. Scotch Pine Teuscher E, Lindequist U, Biogene Gifte - Biologie, Chemie, Pinus species Pharmakologie, 2. Phytopharmaka und the fresh needles, branch tips or fresh twigs is also used pflanzliche Homoopathika, Fischer-Verlag, Stuttgart, Jena, New medicinally, as are the pine tips from fresh and dried shoots. Flower and Fruit: The male flowers are sulfur-yellow in the Brum-Bousquet M et al. The female flowers are purple and Kurihara T, Kikuchi M, (1980) Yakugaku Zasshi 100(10):1054. The buds are reddish, 6 to 12 mm long, From Pinus silvestris: chief components include alpha- oblong-oval and somewhat resinous. The needles are in pairs pinene (10-50%), Delta3-carene (up to 20%), camphene (up and remain on the trees for 3 years. They are various lengths, to 12%), beta-pinene (10-25%), limonene (up to 10%), rigid, twisted, bluish-green with interrupted rows on the additionally including among others myrcene, terpinolene, outside and minimally dentate. Production: Pine shoots (Pini turiones) consist of the fresh or dried, 3 to 5 cm long shoots of Pinus sylvestris. The essential oil Chief components of the raw turpentine oil yielded from (Pini aetheroleum) is obtained from fresh needles, tips of the turpentine from Pinus silvestris include: (-)-alpha-pinene (ca. The oil is recovered form the fresh needles and which and from out of the volatile oils of other pine species branch tips using steam distillation with a successful yield of purified turpentine oil. It must - um rectificatum) is the essential oil obtained from the contain at least 90% pinenes, but no more than 0. Externally, it is used for Ascorbic acid (vitamin C) mild muscular pain and neuralgia. Inhalation should be avoided with • Neuralgias acute inflammation of the breathing passages. Symptoms include albuminuria, diarrhea, dyspnea, • Cough/bronchitis dysuria, feelings of vertigo, hematuria, intestinal colic, • Inflammation of the mouth and pharynx queasiness, reddening of the face, salivation, skin efflores- • Rheumatism cences, sore throat, staggering walk, strangury, thirst, twitching and vomiting. Poisonings can also occur through Purified turpentine oil is used internally and externally for inhalation of the vapors or through skin contact. Externally, the oil is bicarbonate of soda solution, intestinal emptying through used for scabies, burns, frostbite and skin injuries. No health hazards or side effects are known in conjunction Intubation and oxygen respiration may also be necessary. Alcoholic solutions, oils or ointments are used No health hazards are known in conjunction with the proper externally.
In Germany it is known as Mariendistel or Frauendistel buy alendronate 70mg cheap, Chardon-Marie is the common name in French buy alendronate 35 mg online, and cardo mariano and cardo lechero in Spanish. The leaves are alternate, large, white-veined and glabrous with strongly spiny mar- gins. The inforescences are large and round capitula, solitary at the apex of the stem or its branches, surrounded by thorny bracts. The fruits are hard-skinned achenes 6–8 mm long, shiny, generally brownish in colour and with a white silk- like pappus at the apex. According to legend, the white pattern on the leaves represents the breast milk of the Virgin Mary, spilt on the plant while she was breastfeeding Jesus during their escape to Egypt. Milk thistle is native to a narrow area of the Med- iterranean, but has been grown for centuries throughout Europe. The plant was carried to North America by European colonists during the 19th century and is now naturalised in the United States and South America. Formerly cultivated in gardens, it is found in abandoned felds, old pastures and by the roadsides and, in some parts, is considered a problematic invasive weed and a target of classic biological con- trol efforts. Seedling establishment is favourable after rainfalls begin, particularly after a dry summer when there is an absence of grass cover, since thistle seedlings require light to thrive [18]. Each fower head produces approximately 100 seeds, and 10–50 heads are produced per plant, with an av- erage of 3000 seeds per plant, 94 % of them viable. The seeds show little to no dormancy requirements, and any dormancy length is affected by temperature and moisture. The percentage of germination varies from year to year and may be less than 50 % [20]. Milk thistle seeds have after-ripening requirements related to the germination temperature, which lim- its germination to 10–20°C for up to 5 months after harvest. A fairly com- plete list of the chemical compounds of milk thistle can be found in Dr. Duke’s Phytochemical and Ethnobotanical databases and in that author’s handbook [22]. Apart from silymarin, whose components will be described in detail be- low, and other favonolignans, 20–30 % of the fruit is composed of fatty acids, mainly linoleic, linolenic, myristic, oleic, palmitic and stearic acid; 25–30 % is Chapter 6 Silybum marianum (L. According to Duke [22], the following favonoids are also present in the fruit: apigenin, chrysoeriol, eriodictyol, kaempferol, naringenin, quercetin and taxifolin, a synonym for dihydroquercetin. Silymarin belongs to a group of compounds termed favonolignans, and their constituents are formed by an oxidative coupling reaction between the fa- vonoid taxifolin and a phenylpropanoid, usually coniferyl alcohol (Scheme 6. The presence of favonoid-type compounds was observed in milk thistle fruits by Schindler in 1952 [25]. Later on, the research group of Wagner isolated and identifed the hepatoprotective principle of fruits and designated it silyma- rin or silybin [3, 26]. Subsequent work led to the isolation of several other favonolignans as minor constituents of the same and/or different varieties of S. The structures of all favonolignans found in fruits (up to 12) can be found in the revision compiled by Kurkin in 2003 [33]. Corchete From the standpoint of biological activity, silychristin, silydianin and, over- all, silybin (silymarin mixture; Structure 6. During the isolation and structural determination of silybin, its regiosiomer isosilybin was isolated and purifed [27, 34, 35]. Shortly thereafter, it was recog- nised that silybin and isosilybin exist in two diastereoisomeric forms: silybin A and B and isosilybin A and B [34]. The absolute stereochemistries of these compounds have recently been reported by Lee and Liu [36], who fully iso- lated pure isomers from a natural source. According to Freudenberg’s hypothesis [38], which is shared by other au- thors [39–41], the biosynthesis of the favonolignans that contain a 1,4-benzo- dioxane moiety, silybin A and B and isosilybin A and B is most achieved by the reaction between taxifolin and coniferyl alcohol in an oxidative process that is catalysed by peroxidase enzymes. The corresponding radicals are formed frst, followed by their neither regio- nor enantioselective O-coupling. It is not known whether a similar biosynthetic process takes place in the fruits for the synthesis of other favonolignans of the 1,4-benzodi- oxane type or, if so, whether the O-coupling step occurs in an enantioselective manner. This would explain the existence of different chemovariants in the spe- cies, such as, for example, in white-fower varieties, which contain substantial amounts of silandrin and silymonin [43]. The favonolignan content in fruits differs sharply depending on the milk thistle variety in question, the site of developmental growth, and on the cli- matic and physical conditions of the soil since the water and fertilisation re- gime and yield of silymarin range from as low as 0. The individual con- tribution of each compound to the total of the mixture also varies and seems to depend on climate conditions [46]; however, in two inbred generations Hetz et al. The authors concluded that silymarin composition is a genetically fxed character associ- ated with specifc chemoraces. Extraction of silymarin is usually accomplished by defatting the fruits in a soxhlet device with hexane, followed by extraction with organic solvents, mainly acetonitrile, ethanol, ethylacetate or methanol. It has recently been reported that even water at 85–100°C is effective in extracting favonolignans from milk thistle without prior defatting, a procedure outlined in the tradi- tional extraction protocol [48].