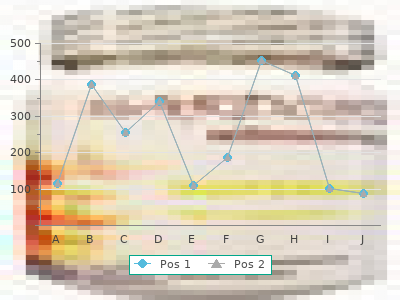
Vasodilan
By H. Taklar. University of Wisconsin-Stout. 2019.
Procainamide delays repolarization and has a greater effect at faster heart rates vasodilan 20mg discount. Dosing Infants/children: Oral: 15 to 30 mg/kg/day safe 20mg vasodilan, divided every 3 to 6 hours (maximum, 4 g/day) I. Procainamide is metabolized in the liver to produce the active metabolite,N-acetyl procainamide 7. Procainamide is moderately dialyzable by hemodialysis, but not dialyzable by peritoneal dialysis. Precautions/Warnings Drug may accumulate in patients with renal or hepatic dysfunction. Long-term administration of procainamide leads to the development of a positive antinuclear antibody test in 50% of patients, which may lead to a lupus erythematosus-like syndrome (in 20–30% of patients). Procaina- mide may potentiate skeletal muscle relaxants; anticholinergic drugs may have enhanced effects. Dubin Compatible Diluents/Administration Do not administer procainamide faster than 20 to 30 mg/min. Mechanism of Action Disopyramide is a potent sodium and potassium channel blocker. Dis- opyramide prolongs the action potential duration in Purkinje tissue more than the effective refractory period. Dosing Infants/children: Oral: Younger than 1 year: 10 to 30 mg/kg/day in four divided doses 1 to 4 years old: 10 to 20 mg/kg/day in four divided doses 4 to 12 years old: 10 to 15 mg/kg/day in four divided doses6 12 to 18 years: 6 to 15 mg/kg/day in four divided doses Adults: Oral (controlled release): If lighter than 50 kg, administer 100 mg every 6 hours, or 200 mg every 12 hours If heavier than 50kg, administer 150mg every 6 hours, or 300mg every 12 hours Adult dose adjustment in renal dysfunction: Clcr 30 to 40 mL/min: 100 mg every 8 hours Clcr 15 to 30 mL/min: 100 mg every 12 hours Clcr less than 15 mL/min: every 24 hours Pharmacokinetics Disopyramide is generally highly protein bound. Disopyramide is metabolized in the liver; its major metabolite has anticholin- ergic and antiarrhythmic effects. Drug-Drug Interactions Hepatic microsomal enzyme-inducing agents (phenytoin, phenobarbital, rifampin) may increase metabolism of disopyramide, and lower serum concentrations. Clarithromycin and erythromycin may increase dis- opyramide concentrations and should not be used with disopyramide. Dubin Lidocaine Indication Lidocaine is used for treatment of ventricular ectopy, tachycardia, and fibrillation; local anesthetic. Mechanism of Action Lidocaine treatment blocks the fast sodium channel and shows frequency dependence. Precautions/Warnings Use lidocaine with caution in patients with hepatic disease, heart failure, hypotension or shock; dose may need to be decreased. Drug-Drug Interactions Cimetidine or β-blockers may increase lidocaine serum levels. Antiarrhythmic Medications 157 Respiratory: respiratory depression or arrest Miscellaneous: allergic reaction (rare) Poisoning Information Lidocaine has a narrow therapeutic index; severe toxicity is seen slightly above the therapeutic range, especially if lidocaine is administered with other antiarrhythmics. Mexiletine Indication Mexiletine is indicated for management of ventriculararrhythmias. Increase dose according to effect Adults: Oral: initial, 200 mg every 8 hours (may load with 400 mg, if necessary). Drug-Drug Interactions Phenobarbital, phenytoin, rifampin, and other hepatic enzyme inducers may lower mexiletine plasma levels. Phenytoin Indication Phenytoin is indicated for ventricular arrhythmias, including those associated with digitalis intoxication and seizures. Phenytoin depresses Phase 4 depolarization, which makes it useful for treating digoxin-induced arrhythmias. Contraindications Heart block and sinus bradycardia are contraindications for phenytoin administration. Drug-Drug Interactions Phenytoin may decrease the effectiveness of ritonavir, valproic acid, ethosuximide, warfarin, oral contraceptives, corticosteroids, etoposide, doxorubicin, vincristine, methotrexate, cyclosporine, theophylline, chloramphenicol, rifampin, doxycycline, quinidine, mexiletine, disopyramide, dopamine, or nondepolarizing muscle relaxants. Serum phenytoin levels may be increased by cimetidine, chloramphenicol, felbamate, zidovudine, isoniazid, trimethoprim, or sulfonamide. Rifampin, zidovudine, cisplatin, vinblastine, bleomycin, antacids, and folic acid may decrease phenytoin levels. Dubin Poisoning Information Symptoms of phenytoin poisoning include unsteady gait, slurred speech, confusion, nausea, hypothermia, fever, hypotension, respiratory depression, and coma. Flecainide Indication Flecainide is indicated for treatment of atrial, junctional, and ven- tricular arrhythmias. Flecainide has a long time constant and takes longer to dissociate from sodium channels. In specialized conduction tissue, refractory periods are shortened and automaticity is decreased. Dosing Children: Oral: initial dose, 1 to 3 mg/kg/day or 50 to 100 mg/m2/day, in three divided doses. Increase every few days to usual 3 to 6 mg/kg/day or 100 to 150 mg/ m2/day in three divided doses, up to 8 mg/kg/day or 200 mg/m2/day Adults: Oral: 50 to 100 mg every 12 hours; increase by 100 mg/day every 4 days.
However purchase vasodilan 20mg line, clotting factors already in the blood- stream continue to coagulate blood until they become depleted cheap 20mg vasodilan free shipping, so anticoagulation doesn’t begin immediately. Patients with this disorder begin taking the medication while still receiving heparin. However, outpatients at high risk for thromboembolism may begin oral anticoagulants without first re- ceiving heparin. To decrease the risk of arterial clotting, oral anticoagulants warfarin levels are sometimes combined with an antiplatelet drug, such as as- pirin, clopidogrel, or dipyridamole. Patients taking warfarin need close monitoring of Drug interactions prothrombin time and In- ternational Normalized Many patients who take oral anticoagulants also receive other Ratios to make sure they drugs, placing them at risk for serious drug interactions. Examples include acetaminophen, allopurinol, amiodarone, If laboratory results fall cephalosporins, cimetidine, ciprofloxacin, clofibrate, danazol, dia- outside the accepted zoxide, disulfiram, erythromycin, fluoroquinolones, glucagon, he- range, warfarin dosage parin, ibuprofen, isoniazid, ketoprofen, methylthiouracil, metron- should be adjusted. Examples include barbiturates, carba- Adverse mazepine, corticosteroids, corticotropin, mercaptopurine, naf- cillin, hormonal contraceptives containing estrogen, rifampin, reactions spironolactone, sucralfate, and trazodone. The primary adverse re- Other interactions include the following: action to oral anticoagu- • A diet high in vitamin K reduces the effectiveness of warfarin. Acute alcohol intoxication increases the ing can occur, however, risk of bleeding. Necrosis or Aspirin, clopidogrel, dipyridamole, sulfinpyrazone, and ticlopi- gangrene of the skin and dine are examples of oral antiplatelet drugs. Quick fix The effects of oral anti- Pharmacokinetics coagulants can be re- When taken orally, antiplatelet drugs are absorbed very quickly versed with phytona- and reach peak concentration in 1 to 2 hours. Sulfinpyra- zone may require several days of administration before its anti- platelet effects occur. The effects of these drugs occur within 15 to 20 minutes of administration and last about 6 to 8 hours. Elderly patients and patients with renal failure may have de- creased clearance of antiplatelet drugs, which would prolong the antiplatelet effect. It lengthens platelet survival and prolongs the patency of arteriovenous shunts used for hemodialysis. Salve for surgery Dipyridamole is used with a coumarin compound to prevent thrombus formation after cardiac valve replacement. Adverse reactions to antiplatelet drugs Hypersensitivity reactions, particularly anaphylaxis, can occur. Tales of toxicity • Aspirin increases the risk of toxicity of methotrexate and val- proic acid. You just don’t know Because guidelines haven’t been established for administrating ticlopidine with heparin, oral anticoagulants, aspirin, or fibrinolyt- ic drugs, these drugs should be discontinued before ticlopidine therapy begins. Pharmacokinetics Direct thrombin inhibitors are typically administered by continu- ous I. They may also be given as an intra-coronary bo- lus during cardiac catheterization. In that case, the drug begins acting in 2 minutes, with a peak response of 15 minutes and a du- ration of 2 hours. In patients with heparin-induced thrombocytopenia, platelet count recovery becomes apparent within 3 days. Bivalirudin and lepirudin are metabolized by the liver and kidneys and excreted in urine Pharmacodynamics Direct thrombin inhibitors interfere with blood clotting by directly blocking all thrombin activity. These drugs offer several advan- tages over heparin: direct thrombin inhibitors act against soluble as well as clot-bound thrombin (thrombin in clots that have al- ready formed); their anticoagulant effects are more predictable than those of heparin; and their actions aren’t inhibited by the platelet release reaction. Also, the dosage of bivalirudin and lepirudin may need Adverse to be reduced in patients with impaired renal function. Use caution when administering a direct thrombin inhibitor to reactions to a patient who has an increased risk of bleeding. Patients at great- bivalirudin est risk for hemorrhage are those with severe hypertension, gas- The major adverse reac- tric ulcers, or hematologic disorders associated with increased tion to bivalirudin is bleeding. Patients receiving spinal anesthesia or those undergoing a lumbar puncture or having major surgery (especially surgery of bleeding; major hemor- the brain, spinal cord, or the eye) also have an increased risk for rhage occurs infre- bleeding. Other adverse reactions include: • intracranial hemor- Drug interactions rhage • Hemorrhage can occur as an adverse reaction to direct throm- • retroperitoneal hemor- bin inhibitors, so avoid giving these drugs with another drug that rhage may also increase the risk of bleeding. Pharmacokinetics Administered subQ, fondaparinux is absorbed rapidly and com- pletely and is excreted primarily unchanged in urine. Its effects peak within 2 hours of administration and last for about 17 to 24 hours. Pharmacotherapeutics Fondaparinux is used only to prevent the formation of blood clots. Adverse reactions to Drug interactions factor Xa Avoid administering fondaparinux with another drug that may in- inhibitors crease the risk of bleeding.
The manufacturer of the drug has yet to publish a detailed report equivalent to those available in the literature on aciclovir and zidovudine cheap 20mg vasodilan visa. Mutagenicity was seen in vitro and in vivo only with high doses of didanosine (Table 1) purchase vasodilan 20mg otc. Didanosine did not induce reverse mutation in Salmonella typhimurium with or without exogenous metabolic activation [no information on doses or strains] and did not induce differential toxicity in Escherichia coli or Bacillus subtilis. Didanosine caused clastogenic effects in Chinese hamster ovary cells and human lymphocytes. Studies of the mutagenicity of didanosine in animals in vivo are limited to assays for micronucleus formation in rodents. As didanosine can be inacti- vated by the low pH of the stomach, it was subsequently administered by intra- peritoneal injection for three consecutive days. A significant clastogenic response was found in peripheral blood at the low and high doses. Over a range of concentrations, the induced mutant frequencies at the two loci were three to four times greater than the values obtained after exposure to didanosine or zidovudine alone. The few available studies on the mutagenicity of didanosine show that it produces primarily clastogenic effects at high doses. It is in widespread use in combination regimens with other antiretroviral agents, and potentiation of the anti- viral effect of didanosine by hydroxyurea is being investigated. About half of the human urinary metabolites are represented by hypoxanthine, and 40% is unchanged drug. Phosphorylation is a minor pathway but is essential for the antiviral activity of the drug. The toxic effects of didanosine in humans include peripheral neuropathy, pancrea- titis, hepatitis and leukopenia. No relevant studies of the reproductive and prenatal effects of didanosine in humans were available. Didanosine crosses the placenta of women and monkeys by bidirectional, passive diffusion. Didanosine but not didanosine triphosphate was observed in placental and fetal tissues. Little information was available on the genetic and related effects of didanosine. Treatment of human cells in culture significantly increased the mutant frequencies after short-term expo- sure to concentrations 10–20-fold greater than the peak plasma concentrations found in some patients. In the same studies, didanosine was more cytotoxic and less muta- genic than zidovudine. There is inadequate evidence in experimental animals for the carcinogenicity of didanosine. Overall evaluation Didanosine is not classifiable as to its carcinogenicity to humans (Group 3). A review of its antiviral activity, pharmaco- kinetic properties and therapeutic potential in human immunodeficiency virus infection. The gelatin capsules may also contain citric acid, gelatin, glycerol, iron oxide, parabens (ethyl and propyl), polyethylene glycol 400, sorbitol and titanium dioxide. Etoposide concentrate for injection is a sterile, non-aqueous solution of the drug in a vehicle, which may be benzyl alcohol, citric acid, ethanol, polyethylene glycol 300 or polysorbate 80. Etoposide phosphate for injection is a sterile, non-pyrogenic, lyophilized powder containing sodium citrate and dextran 40; after reconstitution of the drug with water for injection to a concentration of 1 mg/mL, the solution has a pH of 2. The following impurities are limited by the requirements of The British Pharma- copoeia: 4′-carbenzoxy ethylidene lignan P, picroethylidene lignan P, α-ethylidene lignan P, lignan P and 4′-demethylepipodophyllotoxin (British Pharmacopoeia Commission, 1994). Trade names for etoposide phosphate include Etopofos and Etopophos (Swiss Pharmaceutical Society, 1999). Methods for the analysis of etoposide and its metabolites in plasma, serum and urine have included reversed-phase high-performance liquid chromatography with oxidative electrochemical detection, fluorescence detection and ultraviolet detection. Podophyllotoxin is isolated from the dried roots and rhizomes of species of the genus Podophyllin, such as the may apple or American mandrake (Podophyllin peltatum L. Etoposide can be synthesized from naturally occurring podophyllotoxin by first treating the podophyllotoxin with hydrogen bromide to produce 1-bromo-1-deoxyepi- podophyllotoxin, which is demethylated to 1-bromo-4′-demethylepipodophyllotoxin. The bromine is replaced by a hydroxy group, resulting in 4′-demethylepipodo- phyllotoxin. After protection of the phenolic hydroxyl, the 4-hydroxy group is coupled with 2,3,4,6-tetra-O-acetyl-β-D-glucopyranose. The protecting group at the 4′- hydroxy is removed by hydrogenolysis and the acyl groups by hydrolysis, and the cyclic O-4,6 acetal is formed by reaction with acetaldehyde dimethyl acetal (Holthuis et al. During early clinical trials for cancer chemotherapeutic use, podophyllotoxin proved to be too toxic and, in the 1960s, two epipodophyllotoxins were described, teniposide (see monograph, this volume) and etoposide (Keller-Juslén et al. The first clinical trial of etoposide was reported in 1971, and etoposide entered routine use after 1981 (Oliver et al. Etoposide is one of the most widely used cytotoxic drugs and has strong anti- tumour activity in cases of small-cell lung cancer, testicular cancer, lymphomas and a variety of childhood malignancies. It is one of the most active single agents in the treatment of small-cell lung cancer (Slevin et al.