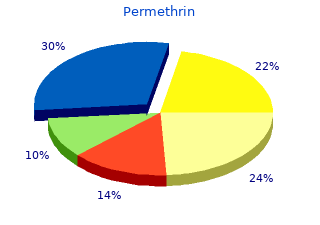
Permethrin
2019, Ohio Northern University, Tjalf's review: "Permethrin 30 gm. Discount online Permethrin no RX.".
For approval of therapy beyond the established maximum duration buy permethrin 30gm line, the prescriber must provide evidence that the patient is engaged in a smoking cessation counseling program discount permethrin 30gm amex. The Fund carries vey data of patient experiences as well as international spending and pricing data. The analysis out this mandate by supporting independent research on health care reveals that Americans, particularly the relatively young and healthy, are more likely to use issues and making grants to improve prescription drugs than are residents of Australia, Canada, Germany, the Netherlands, New health care practice and policy. Support Zealand, and the United Kingdom, but they also experience more financial barriers in access- for this research was provided by ing medications and spend more out-of-pocket for prescriptions. Despite access barriers and disparities, presented here are those of the authors spending per person in the U. Within a generation, prescription drugs have become a major component of health Associate Professor systems worldwide. They are central to most aspects of medicine, from primary care School of Population and Public Health University of British Columbia to specialized treatment. Thus, prescription drugs nearly doubled as a share To learn more about new publications when they become available, visit the of U. As we will discuss, the Americans are generally more likely than residents of experience of several countries shows that a coordinated other surveyed countries to use prescription drugs, national pharmaceutical policy can support achievement according to the 2007 results. Netherlands, New Zealand, the United Kingdom, and Underlying these cross-national differences in 3 reported prescription drug use are patterns of use by age, the United States. We focus our attention in this issue brief on issues of accessibility and cost: health status, and income that reveal potentially impor- tant differences in medical care and equity of access. Pharmaceutical Spending per Person and Growth Rates Total pharmaceutical Pharmaceutical spending spending in U. Prescription Drug Accessibility and Affordability in the United States and Abroad 3 Exhibit 2. Source: Analysis of the Commonwealth Fund 2007 International Health Policy Survey. Differences by Age and health Status may therefore help explain the high use of medicines in The likelihood that older and sicker adults will use pre- the U. For example, Americans age 65 and older are about as likely to use one or more prescriptions per year as simi- equity of Access larly aged persons in the other six countries (Exhibit 2), While cross-national differences in prescription drug use and Americans with two or more chronic conditions are are suggestive of differences in medical practice patterns, about as likely to fill one or more prescriptions as persons differences across population groups within countries with two or more chronic conditions in those countries suggest possible inequities in medical care. The poor are gener- prescription drugs more often than do their counterparts ally less healthy and thus would be expected—with equal in the six other countries. As seen in adults ages 30 to 49 and 50 to 64 are more likely to use Exhibit 4, this expected pattern emerges in five of the at least one prescription than similarly aged people in the seven countries. In Australia, Canada, the Netherlands, other countries, though in the former of these two group- New Zealand, and the U. In the Exhibit 3 shows that Americans with one chronic United States and Germany, however, there was little dif- illness or none were more likely to fill one or more pre- ference between those with below-average income and scriptions than were persons of similar health status in those with average income. Resulting patient requests for prescriptions Americans may be receiving more medicines than they 4 The Commonwealth Fund Exhibit 4. Percent of Population Reporting Use of One or More financial Barriers and Prescription Prescription Drugs During the Previous 12 Months, Drug-Skipping by Country and Income Reported rates of cost-related nonadherence to prescribed All incomes Below-average income treatments add further evidence of inequity in access to Average income Above-average income Percent prescription drugs in the U. With or without adjusting for sex, age, income, income were far more likely than those with above- and health status, residents of all other countries studied average income to rate their health as fair or poor (31% were significantly less likely (50 percent or more) than vs. High- incomes in four of the survey countries have higher rates income Americans were as or more likely to report cost- of use than in this the U. Percent of Population Reporting Not Filling a Prescription or Skipping a Dose Because of Cost During the Previous 12 Months Unadjusted odds ratio Adjusted odds ratio Country rates (95% confdence interval) (95% confdence interval) United States 23. Prescription Drug Accessibility and Affordability in the United States and Abroad 5 Exhibit 6. Average income Above-average income Percent 50 out-of-Pocket Costs 40 Even with their higher rate of unfulfilled prescriptions, 30 Americans are much more likely than residents of the 20 other countries to report out-of-pocket spending in excess of $1,000 in the previous year. The next highest share of population paying $1,000 or more in out-of-pocket for prescription combined in every country except Australia. This likely reflects gaps in cover- In countries with comprehensive drug benefit age and high cost-sharing that even insured Americans programs that have low copayments—Germany, the often experience. Studies repeatedly find negative national differences in drug prices because standard health and total cost effects from high out-of-pocket pre- doses and package sizes vary from country to country scription costs for patients with chronic disease and other and are seldom taken into account in price comparisons. In other countries, a focus on health secure savings has the effect of driving up the list prices and drug benefit policy designed to provide universal of drugs, there is little doubt that uninsured persons in access to essential treatments works together with group the U. Thus, cross-national dif- Affordability of medicines for individual patients is ferences in drug spending likely result from the combined facilitated by policies that limit cost-sharing for covered effects of higher use of medicines in the U. Most of these countries do so with relatively low cost-sharing by Prescription Drug Accessibility and Affordability in the United States and Abroad 7 patients, especially for vulnerable populations (e. Such comparative assessment review can help spur Canadian system of public drug coverage is comparable both the development and adoption of innovative and to that of the U. However, public programs for coverage under a universal drug benefit system, a finance a greater share of total prescription drug costs in key consideration is the price that can be charged. In Canada, prices are limited in com- to geography, age, income, or employment—can be parison to those charged in seven comparator countries cost-effective when viewed from health system and (including the U.
They may be impossible to distinguish at points of care from the genuine product and may lead to under-dosage and high levels of treatment failure permethrin 30gm with mastercard, giving a mistaken impression of resistance buy cheap permethrin 30gm, or encourage the development of resistance by providing sub-therapeutic blood levels. Substandard drugs result from poor-quality manufacture and formulation, chemical instability or improper or prolonged storage. Artemisinin and its derivatives in particular have built-in chemical instability, which is necessary for their biological action but which causes pharmaceutical problems both in their manufacture and in their co-formulation with other compounds. The requirement for stringent quality standards is particularly important for this class of compounds. Many antimalarial drugs are stored in conditions of high heat and humidity and sold beyond their expiry dates. In many malaria-endemic areas, a large proportion of the antimalarial drugs used are generic products purchased in the private sector. They may contain the correct amounts of antimalarial drug, but, because of their formulation, are inadequately absorbed. Antimalarial medicines must be manufactured according to good manufacturing practice, have the correct drug and excipient contents, be proved to have bioavailability that is similar to that of the reference product, have been stored under appropriate conditions and be dispensed before their expiry date. Legal and regulatory frameworks must be strengthened, and there should be greater collaboration between law enforcement agencies, customs and excise authorities and medicines regulatory agencies to deal more effectively with falsifed medicines. Private sector drug distribution outlets should have more information and active engagement with regulatory agencies. Manufacturers of antimalarial medicines with prequalifed status are listed on the prequalifcation web site. Good practice statement When adapting and implementing these guidelines, countries should also strengthen their systems for monitoring and evaluating their national programmes. The systems should allow countries to track the implementation and impact of new recommendations, better target their programmes to the areas and populations at greatest need and detect decreasing antimalarial effcacy and drug resistance as early as possible. In the “test, track, treat” initiative, it is recommended that every suspected malaria case is tested, that every confrmed case is treated with a quality-assured antimalarial medicine and that the disease is tracked by timely, accurate surveillance systems. Surveillance and treatment based on confrmed malaria cases will lead to better understanding of the disease burden and enable national malaria control programmes to direct better their resources to where they are most needed. An antimalarial medicine that is recommended in the national malaria treatment policy should be changed if the total treatment failure proportion is ≥ 10%, as assessed in vivo by monitoring therapeutic effcacy. A signifcantly declining trend in treatment effcacy over time, even if failure rates have not yet fallen to the ≥ 10% cut-off, should alert programmes to undertake more frequent monitoring and to prepare for a potential policy change. However these early relapses (or any newly acquired infections) should be suppressed by therapeutic doses of slowly eliminated antimalarial drugs such as chloroquine, mefoquine and piperaquine. Reappearance of parasitaemia within 28 days of treatment (whether relapse, recrudescence or re-infection) can therefore still be used as a proxy measure of resistance. Resistance to antimalarial drugs arises because of selection of parasites with genetic changes (mutations or gene amplifcations) that confer reduced susceptibility. Resistance has been documented to all classes of antimalarial medicines, including the artemisinin derivatives, and it is a major threat to malaria control. Widespread inappropriate use of antimalarial drugs exerts a strong selective pressure on malaria parasites to develop high levels of resistance. Resistance can be prevented or its onset slowed considerably by combining antimalarial drugs with different mechanisms of action and ensuring high cure rates through full adherence to correct dose regimens. If different drugs with different mechanisms of resistance are used together, the emergence and spread of resistance should be slowed. Clinical and parasitological assessment of therapeutic effcacy should include: • confrmation of the quality of the antimalarial medicines tested; • molecular genotyping to distinguish between re-infections and recrudescence and to identify genetic markers of drug resistance; • studies of parasite susceptibility to antimalarial drugs in culture; and • measurement of antimalarial drug levels to assess exposure in cases of slow therapeutic response or treatment failure. Good practice statement When possible: • use fxed-dose combinations rather than co-blistered or loose, single- agent formulations; and • for young children and infants, use paediatric formulations, with a preference for solid formulations (e. Good practice statement These guidelines provide a generic framework for malaria diagnosis and treatment policies worldwide; however, national policy-makers will be required to adapt these recommendations on the basis of local priorities, malaria epidemiology, parasite resistance and national resources. Broad, inclusive stakeholder engagement in the design and implementation of national malaria control programmes will help to ensure they are feasible, appropriate, equitable and acceptable. Transparency and freedom from fnancial conficts of interest will reduce mistrust and confict, while rigorous evidence-based processes will ensure that the best possible decisions are made for the population. In some countries, the group adapting the guidelines for national use might have to re-evaluate the global evidence base with respect to their own context. Failure to implement the basic principles of combination therapy and rational use of antimalarial medicines will risk promoting the emergence and spread of drug resistance, which could undo all the recent gains in malaria control and elimination. High-quality light microscopy requires well- trained, skilled staff, good staining reagents, clean slides and, often, electricity to power the microscope. It requires a quality assurance system, which is often not well implemented in malaria-endemic countries. In many areas, malaria patients are treated outside the formal health services, e. Where possible, however, blood smears should be examined by microscopy, with frequent monitoring of parasitaemia (e. Although there are minor differences in the oral absorption, bioavailability and tolerability of the different artemisinin derivatives, there is no evidence that these differences are clinically signifcant in currently available formulations. It is the properties of the partner medicine and the level of resistance to it that determine the effcacy of a formulation.
I feel great gratitude to my mother Liisa and father Heikki for their love and support throughout my life discount 30gm permethrin overnight delivery. My sisters Maarit and Pauliina and their husbands Panu and Yrjö are thanked for their help and support generic permethrin 30gm otc. My parents-in-law Tarja and Markus also deserve warm thanks for their interest in my work. My brother-in-law Esa and his wife Anniina are thanked for sharing these years with me. Finally, my deepest and the most sincere thanks go to my dearly beloved wife Mirja for her support and understanding during these years and for taking care of our wonderful son Rasmus, who has filled my life with happiness. In the published version of abstract (results) the number of operations should be “3. A corrected version of the table is displayed below: The patients underwent an average of 3. The unit in the legend should be “months” instead of “years” in the published version of Figure 4. We ensure there is solid involving over 100 companies who are major generators of carbon dioxide in Ireland. Wexford, Ireland Telephone: + 353 53 9160600 Fax: + 353 53 9160699 Email: info@epa. Neither the Environmental Protection Agency nor the author(s) accept any responsibility whatsoever for loss or damage occasioned or claimed to have been occasioned, in part or in full, as a consequence of any person acting or refraining from acting, as a result of a matter contained in this publication. All or part of this publication may be reproduced without further permission provided the source is acknowledged. John Fitzgerald, Inspector, Department of Environment, Community and Local Government Mr. Peter O’Reilly, Senior Engineer, Fingal County Council (representing the Water Services Training Group) Mr. The Environmental Protection Agency was established in 1993 to licence, regulate and control activities for the purposes of environmental protection. In the Environmental Protection Agency Act, 1992 (Section 60), it is stated that “the Agency may, and shall if so directed by the Minister, specify and publish criteria and procedures, which in the opinion of the Agency are reasonable and desirable for the purposes of environmental protection, in relation to the management, maintenance, supervision, operation or use of all or specified classes or plant, sewers or drainage pipes vested in or controlled or used by a sanitary authority for the treatment of drinking water…. This manual has been prepared to reflect best practice in drinking water disinfection. Daily log sheets for operators of disinfection equipment for the verification of disinfection system operation. Source waters, susceptible to surface contamination, particularly surface waters and groundwater and spring sources contain micro-organisms such as bacteria, viruses and protozoan parasites (e. Cryptosporidium) which can present a risk to human health if not effectively treated and disinfected. The overriding objective of water treatment is the removal or inactivation of pathogenic micro-organisms to prevent the spread of waterborne disease. It is important that water treatment works be equipped with adequate disinfection systems, when pristine water supplies collected from catchments totally under the control of the water supply authority are now a rarity. Removal of pathogenic organisms is effected by processes involving addition of coagulant chemicals followed by sedimentation and filtration and by other filtration processes such as membrane filtration. In contrast to removal, the concept of inactivation of pathogens in water relates to the effect that the application of a disinfectant has in destroying the cellular structure of the micro-organisms or in disrupting its metabolism, biosynthesis or ability to grow/reproduce. In the case of bacteria, inactivation describes the subsequent inability of the microorganism to divide and form colonies. For viruses, inactivation measures the inability of the microorganism to form plaques in host cells. For protozoan Cryptosporidium oocysts, it measures the inability of the microorganism to multiply, thereby preventing consequent infection of a host by Cryptosporidium. The philosophy underlying disinfection of all water supplies is to use the best quality source of water available and to provide multiple barriers to the transmission of any pathogenic organisms to consumers. Objective of the updated manual The objective of this disinfection manual is to provide practical guidance and information to the following: a) Water Service Authorities and Private Water Suppliers to allow them to design and operate water treatment systems to provide rigorous disinfection, whilst maintaining compliance with other water quality parameters, particularly in relation to disinfection by-products. This Guidance Manual does not deal with the hazards posed by the generation, storage or use of these chemicals in water treatment or disinfection, the interaction of these chemicals or the associated risks for plant operators Water Treatment Manual: Disinfection managing the production of drinking water for Water Service Authorities or private drinking water suppliers. The Safety, Health and Welfare Act 2005 addresses the responsibilities of Water Service Authorities and private suppliers in the management of these operator risks. Regulation 5 stipulates that “measurement of compliance with the parametric values specified in Part 1 of the Schedule shall be made in the case of— (a) water supplied from a distribution network or a private source, at the point within a premises at which it emerges from the tap or taps that are normally used for the provision of water for human consumption; (b) water supplied by tanker or similar means, at the point at which it emerges from it; (c) water used in a food-production undertaking, at the point where the water is used in the undertaking. Regulation 4 directs that “Water shall be regarded as wholesome and clean if - (a) it is free from any micro-organisms and parasites and from any substances which in numbers or concentrations, constitute a potential danger to human health, and (b) it meets the quality standards specified …. Regulation 7 (10) stipulates that the Supervisory Authority shall ensure “additional monitoring is carried out on a case-by-case basis (whether by itself or the relevant water supplier) of substances and micro-organisms for which no parametric value has been specified in Part 1 of the Schedule, if there is reason to suspect that such substances or micro-organisms may be present in amounts or numbers that constitute a potential danger to human health” Water Treatment Manual: Disinfection and may issue direction to a supplier where it is of the “opinion that— (a) non-compliance with a water quality standard or other parametric value specified in Part 1 of the Schedule, or (b) the presence of any substance or micro-organism for which no water quality standard has been prescribed, in water intended for human consumption, or the inefficiency of related disinfection treatment, constitutes, or may constitute, a risk to human health” C. Regulation 9 requires that if Water Service Authorities “… in consultation with the Health Service Executive, considers that a supply of water intended for human consumption constitutes a potential danger to human health, the authority shall…. Regulation 13 sets out as follows the obligations of Water Service Authorities and regulated Private Water Suppliers with respect to the monitoring and verification of disinfection systems; “where disinfection forms part of the preparation or distribution of water intended for human consumption, the efficiency of the disinfection treatment is verified and that any contamination from disinfection by-products is kept as low as possible without compromising the disinfection, in accordance with such directions as the relevant supervisory authority may give”. However many of these disinfectant chemicals if overdosed or used inappropriately, as part of a water treatment process, can result in the formation of disinfection by-products.
Radical (or total) prostatectomy is the removal of the entire prostate and some of the tissue around it safe 30 gm permethrin. Radiation may come from a machine outside the body (external beam radiation therapy) purchase 30 gm permethrin otc. Or, it may come from radioactive material placed in the body near cancer cells (internal radiation therapy or brachytherapy). Standard therapy: Treatment that experts agree is appropriate, accepted, and widely used. It is needed to develop and maintain male sex characteristics, such as facial hair, deep voice, and muscle growth. Tumor: An abnormal mass of tissue that results when cells divide more than they should or do not die when they should. It is used in conditions that progress slowly, are hard to diagnose, or may get better without treatment. Drug interactions to look out for (because of risk of bradycardia/atrioventricular block): o Verapamil, diltiazem (should be discontinued). Low heart rate: • If <50 bpm and worsening symptoms, halve dose of beta-blocker, or, if severe deterioration, stop beta-blocker (rarely necessary). Asymptomatic low blood pressure: • Does not usually require any change in therapy. Symptomatic hypotension: • If dizziness, light headedness, or confusion and a low blood pressure, reconsider need for nitrates, calcium-channel blockers , and other vasodilators andb reduce/stop, if possible. Note: beta-blockers should not be stopped suddenly unless absolutely necessary (there is a risk of a ‘rebound’ increase in myocardial ischaemia or infarction and arrhythmias). K+-sparing diuretics such as amiloride and triamterene) and nephrotoxic agents (e. To relieve breathlessness and oedema in patients with symptoms and signs of congestion. Thaizde diuretics can be used in patients with preserved renal function and mild symptoms of congestion. Symptomatic or severe asymptomatic hypotension (systolic blood pressure <90 mmHg) – may be made worse by diuretic-induced hypovolaemia. Use minimum dose necessary to maintain euvolaemia – the patient’s ‘dry weight’ (i. Symptomatic hypotension: • Causing dizziness/light headedness – reduce dose if no symptoms or signs of congestion. Hyponatraemia: • Volume depleted: o Stop thiazide or switch to loop diuretic, if possible. Hypovolaemia/dehydration: • Assess volume status; consider diuretic dosage reduction. Severe liver dysfunction or renal dysfunction (no evidence on safety or pharmacokinetics for creatinine clearance <15 mL/min). Double the dose not more frequently than at 2-week intervals (slower up-titration may be needed in some patients). Aim for target dose (see above) or, failing that, the highest tolerated dose based on resting heart rate. If the resting heart rate is between 50 and 60 bpm, the current dose should be maintained. However, if they result in the patient’s discomfort, the discontinuation of ivabradine should be considered. Side effects due to symptomatic bradycardia: breathlessness, fatigue, syncope, dizziness; other side effects: luminous visual phenomena. This document presents a comprehensive review of the best available evidence up to January 2010, examining the effcacy of a broad range of psychological interventions across the mental disorders affecting adults, adolescents and children. While every reasonable effort has been made to ensure the accuracy of the information, no guarantee can be given that the information is free from error or omission. Such damages include, without limitation, direct, indirect, special, incidental or consequential. Apart from any use permitted under the Copyright Act 1968, no part may be reproduced without prior permission from the Australian Psychological Society. Delivery of evidence-based > Generalised anxiety disorder psychological interventions by appropriately trained > Panic disorder mental health professionals is seen as best practice > Specifc phobia for Australian psychological service delivery. Therefore, > Social anxiety disorder keeping abreast of new developments in the treatment > Obsessive compulsive disorder of mental disorders is crucial to best practice. The body of evidence-based research > Bulimia nervosa will continue to expand over time as the barriers to > Binge eating disorder conducting systematic evaluations of the effectiveness of various interventions are identifed and new Adjustment disorder research methodologies are developed. Sexual disorders This review builds on the earlier literature review by expanding the list of mental disorders to include Somatoform disorders posttraumatic stress disorder, social anxiety, and > Pain disorder somatoform disorders.