Lansoprazole
By H. Irmak. Lincoln University, San Francisco California.
Other relevant documents as prescribed in note 1 of Yes/No Form 27C Opinion: All the relevant documents submitted along with the forwarding letter of S buy lansoprazole 15mg on-line. Any action for contravention of section 10 of the Drugs and Cosmetics Act is resorted to by advising the Commissioner of Customs to take action under section 11 of Drugs & Cosmetics Act cheap lansoprazole 15mg mastercard, read with relevant provisions of customs Act 1962. All the port offices are headed by Assistant Drugs Controller (India) and assisted by Technical Officers/Drugs Inspectors along with some ministerial staff members. Requirements and check list for import of drugs (The documents required to be submitted by the importer and exporter should be displayed in the official notice board for perusal of the applicants and common public. No registration certificate is required for non-critical in- vitro diagnostic kits and reagents (Rule 24(2)) and inactive bulk substances (Rule 24 A (8)) However Form 10 is required for non critical invitro diagnostic kits and reagents. A Registration Certificate (Form 41), unless, it is sooner suspended or cancelled, shall be valid for a period of three years from the date of issue: provided that if the application for a fresh Registration Certificate is made nine months before the expiry of the existing certificate, the current registration certificate shall be deemed to continue in force until orders are passed on the application. Small quantities of new drugs the import of which is otherwise prohibited under section 10 of the act may be imported by a Govt. Hospital or Autonomous Medical Institution for the treatment of patients under a license in form 11-A(Rule 33-A). Patent and proprietary medicines shall be imported only in containers intended for retail sale. No drug having the shelf life of less than 60% is allowed for the import: provided that in exceptional cases the licensing authority may, for the reasons to be recorded in writing, allow the import of any drug having lesser shelf life period, but before the date of expiry as declared on the container of the drug (Rule 31). No drug, the manufacture, sale or distribution of which is prohibited in the country of origin shall be allowed to be imported under the same name or under any other name (Rule 30 B). All the drugs imported in India are required to be stored at drug/product specific temperature conditions. All the drugs imported should comply to the standards as specified in the Second Schedule to the Act and Rules there under. All the drugs/formulations imported into the India shall fulfil the labelling requirements as prescribed in Drugs and Cosmetics Rules1945. Self Certified copy of Form10 or 10A and Registration Certificate (Form 41) as the case may be 2. Original license in Form 11/11-A to make the debit for the quantity imported under respective bill of entry. If goods are not directly supplied from the manufacturer then the port officer may verify the authenticity of goods at manufacturer‘s end through e-mail/fax or his authorized registered agent in India. After scrutiny of the aforesaid documents and making the necessary entry in the records/computer, the technical staff to put up the Bill of entry (B/E) to the port officer. The Port officer should examine B/E and should decide at this stage whether:- a) Labelling & marking need to be checked by the port officers and samples may be drawn (If the drug imported is in small container of 5 kg or less than the original container may be called for to check the markings/label) b) When required Samples to be sent for testing / analysis to the Government / Approved testing lab. However, the port officer may draw more samples depending on the previous test reports, number of consignments and the reputation of the manufacturer/ importer. There are no proper labels/markings or no markings on the containers or the markings are illegible. Drugs imported from a supplier/manufacturer have been reported to be not of standard quality/spurious etc at this port or any other port in India. The price of the drug imported is abnormally low as compared with the previous imports. Pending testing report, to avoid demurrage if the importer gives an undertaking (Rule 40 (1)) in writing not to dispose of the drugs without the consent of Customs commissioner etc. Drugs requiring cold storage such as sera, vaccines, may be released forthwith conditionally on L/G for test etc. If there are any labelling defects and importer desire to rectify the defects at their place, they may be allowed to be clear the consignment on L/G for rectification of labelling and/or test. Samples are drawn as far as possible under the direct supervision of a technical representative of the port office. Also, sampling should invariably be carried out in the presence of the importer‘s representative. In case of drugs requiring special precautions due to their hygroscopic, thermo labile nature etc. If the drug is sterile, the importers should be asked to make arrangement for drawing of samples under sterile conditions. Usually √n+1 number of 417 samples may be drawn, where n is number of containers / batches as per requirements. No samples should be drawn from the consignments imported for the purpose of registration only. It is responsibility of the Port Officer to ensure that all samples intended for test, are sent to laboratory as early as possible.
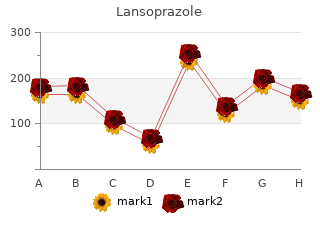
On the basis of 8699 patient–years of follow-up and an annual incidence rate of 3–4 cases of acute myeloid leukaemia per 100 000 population (Parkin et al order lansoprazole 15mg amex. According to Bokemeyer and Schmoll (1995) cheap 15 mg lansoprazole, the cumulative risk for acute myeloid leukaemia was only 0. Cohort studies of other types of cancer are summarized in Table 4 and are described below. The expected number of cases of acute myeloid leukaemia in the general population can be approximated from a world standardized incidence rate of 4–5 per 100 000 persons (see text). After having achieved complete remission, the patients received maintenance treatment with epipodophyllotoxins according to seven schedules (Table 5): 580 patients received teniposide (see monograph, this volume), and a substantial proportion of these (301) also received etoposide. In addition, most patients received methotrexate, mercaptopurine, prednisone, vincristine, asparaginase and cytarabine, and some patients received cyclophosphamide, doxorubicin and cranial irradiation. Acute myeloid leukaemia developed in 21 children (as a first adverse event in 17), with an overall cumulative risk of 3. The median interval between the diagnoses of acute lymphoblastic leukaemia and acute myeloid leukaemia was 40 months. Six of the cases were acute myelomonocytic leukaemia, eight were acute monoblastic leukaemia, three were acute myeloblastic leukaemia, one was acute mega- karyoblastic leukaemia, one was acute myeloid leukaemia and two were acute undiffer- entiated leukaemia. In four patients, acute myeloid leukaemia developed after relapse had occurred, and these were not included in the statistical analyses. In the analysis of leukaemia risk, the doses of teniposide and etoposide were weighted equally, since the potency of teniposide in vitro—10 times that of etoposide—is offset in vivo by exten- sive protein binding, resulting in 10 times less unbound (active) drug (see section 4). The schedule of epipodophyllotoxin treatment appeared to be a crucial factor in deter- mining the risk for acute myeloid leukaemia, as the strongest evidence was obtained by comparing two subgroups that differed only in their schedule of epipodophyllotoxin administration. The multivariate analysis indicated that the frequency of epipodophyllotoxin administration was a much more important determinant of risk for acute myeloid leukaemia than cumulative dose. The induction and maintenance treatment consisted of prednisone, vincristine, dauno- rubicin, asparaginase, methotrexate, mercaptopurine, leucovorin, intravenous etoposide (300 mg/m2) and cytarabine. The first 33 patients received teniposide instead of eto- poside at half the dose. Ten children developed secondary acute myeloid leukaemia, two of which were of the myelomonocytic type and two of the monoblastic type; one developed myelodysplastic syndrome (consistent with chronic myelomonocytic leukaemia), and one had refractory anaemia with excess blasts in transformation. The interval between the diagnosis of acute lymphoblastic and acute myeloid leukaemia ranged from 23 to 68 months. The median dose of etoposide administered was 7900 mg/m2 (range, 5100–9900 mg/m2). One child with acute myeloid leukaemia had received teniposide instead of etoposide. The Working Group also noted that it was not completely clear in these two studies whether the diagnosis of acute lympho- blastic leukaemia excluded primary mixed leukaemia and thus allowed differentiation of lymphoblastic from myeloid disease. A total of 465 children [ages not given] with primary rhabdomyosarcoma (diagnosis around 1984) took part in this trial. The analysis was restricted to 207 children who had survived more than 36 weeks from entry into the protocol. They had received etoposide daily in combination with two courses of dactinomycin (cumulative dose of etoposide, 600 mg/m2) or three courses of cisplatin (cumulative dose of etoposide, 900 mg/m2), after they had been treated with induction regimens that included cyclophosphamide and doxorubicin. Interim analyses of the risks for acute myeloid leukaemia and myelodysplastic syndrome were carried out when four cases had been observed. Two of the four cases had received etoposide (600 mg/m2) and dactino- mycin, and two had received etoposide (900 mg/m2) and cisplatin. The three cases of acute myeloid leukaemia were of the myelomonocytic and monoblastic types and myelodysplastic syndrome progressing to acute myeloid leukaemia; the other case was myelodysplastic syndrome. The calculated cumulative six- year rate of development of acute myeloid leukaemia or myelodysplastic syndrome was 3. Twelve trials were selected from a pool of approximately 100 in which etoposide or teniposide had been used. Selection was made without knowledge of the number of secondary leukaemias that had occurred to date in the trials. The 12 trials (11 for patients with solid tumours and one for patients with acute lymphoblastic leukaemia) were divided into three strata according to the cumulative dose of eto- poside: low (< 1500 mg/m2), moderate (1500–3000 mg/m2) and high (> 3000 mg/m2). For trials in which teniposide was used, a 1:2 ratio was used to convert the dose of teniposide to that of etoposide. Patients treated with the low dose had primary rhabdo- myosarcoma (n = 222) or medulloblastoma (advanced stage) (n = 229). The patients with rhabdomyosarcoma had also received cyclophosphamide or equivalent doses of ifosfamide (25 000–35 000 mg/m2). Patients treated with the moderate dose had primary neuroblastoma (n = 319), germ-cell tumour (adult and paediatric) (n = 700) or acute lymphoblastic leukaemia (high risk) (n = 251). Patients given the higher dose had primary rhabdomyosarcoma (n = 313) or Ewing sarcoma (n = 257). They also received cyclophosphamide or equivalent doses of ifosfamide (25 000– 35 000 mg/m2). The six-year actuarial risks for acute myeloid leukaemia or myelo- dysplastic syndrome were 3.
Adverse reactions • Common: diarrhea (47%) purchase 15mg lansoprazole overnight delivery, abdominal cramping (14%) cheap lansoprazole 30mg without a prescription, nausea, vomiting, rash (24%), pruritus (17%), stomatitis. Clinically important drug interactions • Drugs that increase effects/toxicity of auranofin: penicillamine, immunosuppressants, cytotoxic drugs. Editorial comments: This drug is not listed in the Physician’s Desk Reference, 54th edition, 2000. Editorial comments • Usually available in combination with other agents, including pseudoephedrine, phenylephrine, and phenylpropanolamine. Warnings and precautions, side effects, etc, of other ingredients should be kept in mind when prescribing. Adjustment of dosage • Kidney disease: creatinine clearance 10–50 mL/min: reduce dose by 25%; creatinine clearance <10 mL/min: reduce dose by 50%. Do not discontinue unless no effect occurs after 3 months of use for rheumatoid arthritis. Warnings/precautions • Use with caution in patients with the following conditions: liver and renal disease. Advice to patient • Use two forms of birth control including hormonal and barrier methods. Editorial comments • Because of risk of severe bone marrow depression, frequent monitoring of complete blood counts and platelet counts are recommended. It is suggested that these should be performed weekly for the first month, twice monthly for the second and third months, and then monthly thereafter. Discontinuation of the drug is recommended if there is rapid development of leukopenia, thrombocytopenia, or other signs of bone marrow depression. Ran- domized, double blind placebo controlled trials have demon- strated efficacy of 6-mercaptopurine and azathioprine in active or quiescent Crohn’s disease. Patients in these trials were often able to taper prednisone doses to 5 mg/d or less. Use latex gloves and safety glasses when han- dling parenteral form of this medication. Susceptible organisms in vivo: Like erythromicin but less active against gram-positive bacteria and more active against gram- negative bacteria. Clinically important drug interactions • Drugs that decrease effects/toxicity of macrolides: rifampin, antacids (aluminum, magnesium). Parameters to monitor • Signs and symptoms of superinfection, in particular pseudomem- branous colitis. Editorial comments • Azithromycin has the advantage of improved compliance com- pared with erythromycin because of better tolerability, daily dosage, and shorter course of therapy. Susceptible organisms in vivo: staphylococci, Streptococcus pneumoniae, beta-hemolytic streptococci, Streptococcus faecalis, Streptococcus viridans, Escherichia coli, Hemophilus influen- zae, Neisseria gonorrhoeae, Proteus mirabilis, Salmonella sp, Shigella sp. Children >25 kg: 50 mg/kg/d, two equal doses • Gonorrhea, acute uncomplicated urogenital infections Adults: 1. Editorial comments • Bacampicillin has no advantage over ampicillin and has a sim- ilar spectrum of activity. Mechanism of action: Inhibits mono- and polysynaptic reflexes within the spinal cord resulting in decreased spasticity. Warnings/precautions • Use with caution in patients with the following conditions: seizures, decreased renal function. Sit at the edge of the bed for several minutes before standing, lie down if feeling faint or dizzy. Male patients with orthostatic hypotension may be safer urinating while seated on the toilet rather than standing. Editorial comments: Balclofen appears to also be an effective treatment for refractory hiccups (singultus). Mechanism of action: Inhibits elaboration of many of the medi- ators of allergic inflammation, eg, leukotrienes and other prod- ucts of the arachidonic acid cascade. Contraindications: Untreated fungal, bacterial, or viral infec- tions, untreated infections of nasal mucosa, hypersensitivity to corticosteroids. Warnings/precautions • Use with caution in patients with the following conditions: tuberculosis of the respiratory tract (active or quiescent), expo- sure to measles or chicken pox. Alternatively, adrenal insufficiency may occur: weakness, fatigue, nausea, anorexia. This may minimize the development of dry mouth, hoarseness, and oral fungal infection. Adverse reactions • Common: nasal irritation, cough, pharyngitis, sneezing attacks. Parameters to monitor • Signs and symptoms of acute adrenal insufficiency, particu- larly in response to stress. If these occur, the dose of systemic steroid should be increased followed by slower withdrawal.
Each joint which a claim is made; and should be clamped together to ensure a com- (9) A statement of the sections of the plete seal throughout the analysis safe 30mg lansoprazole. The sepa- regulations for which an exemption is ratory funnel 30mg lansoprazole with amex, B, should have a capacity of sought. The proposal with sulfur dioxide, is deposited in the funnel should be clearly identified as a re- and the side arm. The gas inlet tube, D, (Kontes K– labeling experiments and submitted as 179000 or equivalent) should be of sufficient a citizen petition under §10. The bubbler, F, was fabricated from Foods labeled in violation of existing glass according to the dimensions given in regulations will be subject to regu- Fig. Just prior to use, small enough to pass through the 24/40 point add three drops of methyl red indicator and of flask C. Wipe the tapered (e) Nitrogen—A source of high purity nitro- joint clean with a laboratory tissue, apply gen is required with a flow regulator that stopcock grease to the outer joint of the will maintain a flow of 200 cc per minute. To separatory funnel, and return the separatory guard against the presence of oxygen in the funnel, B, to tapered joint flask C. The nitro- nitrogen, an oxygen scrubbing solution such gen flow through the 3% hydrogen peroxide as an alkaline pyrogallol trap may be used. Close Use a power setting which will cause 80 to 90 the stopcock of separatory funnel, B, and add drops per minute of condensate to return to 90 ml of 4N hydrochloric acid to the sepa- the flask from condenser, E. Begin the flow of nitrogen at of boiling the contents of the 1000 ml flask a rate of 200±10 cc/min. Compute the sulfite minutes the apparatus and the distilled content, expressed as micrograms sulfur di- water will be thoroughly de-oxygenated and oxide per gram of food (ppm) as follows: the apparatus is ready for sample introduc- tion. Add 100 ml of 5% endpoint; the factor, 1000, converts milli- ethanol in water and briefly grind the mix- equivalents to microequivalents and ture. Grinding or blending should be contin- Wt=weight (g) of food sample introduced into ued only until the food is chopped into pieces the 1000 ml flask. The following requirements shall Nonstandardized Foods apply unless modified by a specific reg- ulation in subpart B of this part. For each characterizing ingre- dient or component, the words "l per- Subpart A—General Provisions cent or %) lll" shall appear fol- lowing or directly below the word §102. The distinct contrast to other printed or name shall be uniform among all iden- graphic matter, and in a height not tical or similar products and may not less than the larger of the following al- be confusingly similar to the name of ternatives: any other food that is not reasonably (i) Not less than one-sixteenth inch encompassed within the same name. A neous impression that such ingre- petition requesting such a regulation, dient(s) or component(s) is present which would amend the applicable reg- when it is not, and consumers may oth- ulation, shall be submitted pursuant to erwise be misled about the presence or part 10 of this chapter. Specific Nonstandardized Foods (1) The presence or absence of a char- acterizing ingredient or component §102. The names "hydrolyzed vege- easily legible boldface print or type in table protein" and "hydrolyzed pro- distinct contrast to other printed or tein" are not acceptable because they graphic matter, and in a height not do not identify the food source of the less than the larger of the alternatives protein. The availability of this in- weight of the finished product, and the corporation by reference is given in overall biological quality of the pro- paragraph (c)(1) of this section. The availability of this in- the finished product, and the overall corporation by reference is given in biological quality of the protein con- paragraph (c)(1) of this section. This part of the corporation by reference is given in name shall be placed immediately fol- paragraph (c)(1) of this section. The word "frozen" pared by use of the package contents is also optional, provided that the (e. The 5-percent range, when used, shall be declared in the The common or usual name of a mix- manner set forth in §102. The term shall be in a a line(s) immediately below the words type size no less than one-half the "onion rings" in easily legible boldface height of the letters in the name of the print or type in distinct contrast to juice. However, if water is added to (2) Not less than one-half the height this 100 percent juice mixture to adjust of the largest type used in the words the Brix level, the product shall be la- "onion rings. I (4–1–10 Edition) the nutritional quality guideline estab- the guideline, shall be ineligible to lished for its class of food may state bear the guideline statement provided "This product provides nutrients in for in paragraph (b) of this section, and amounts appropriate for this class of such a product shall also be deemed to food as determined by the U. Govern- be misbranded under the act unless the ment," except that the words "this label and all labeling bear the fol- product" are optional. This statement, lowing prominent and conspicuous if used, shall be printed on the prin- statement: "The addition of lll to cipal display panel, and may also be (or "The addition of lll at the level printed on the information panel, in contained in) this product has been de- letters not larger than twice the size of termined by the U. Government to be the minimum type required for the unnecessary and inappropriate and declaration of net quantity of contents does not increase the dietary value of by §101. Labeling of the food," the blank to be filled in with noncomplying products may not in- the common or usual name of the nu- clude any such statement or otherwise trient(s) involved. It tween a product to which nutrient ad- could also result in deceptive or mis- dition has or has not been made in leading claims for certain foods. The order to meet the guideline, except Food and Drug Administration does that a nutrient addition shall be de- not encourage indiscriminate addition clared in the ingredient statement.