Minocycline
By Y. Gembak. Texas A&M University, Commerce. 2019.
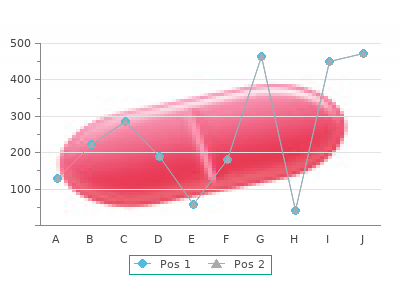
A ntich olinergicoveractive bladdersyndrom e drugs com pared with oth erdrugs A uth or cheap minocycline 50mg with amex, A dverse effects assessed? Y ear H ow assessed W ith drawals due to adverse events H ycoscyam ine Serels1998 Percentages are inorder:H yo safe 50 mg minocycline,Dox,combination N otR eported M oderate-to-severesideeffects:19(61%),8(47%),8(61%) Thesepercentagesareestim atedfrom agraph: D ry m outh:70%,20%,58% F atigue:33%,31%,8% D iz z iness:25%,20%,23% Headaches:22%,8%,8% Constipation:26%,11%,8% D iarrhea:10%,8%,0% Vaginaldry ness:3%,0%,0% N ightsweats:3%,0%,0% L eg edem a:0%,3%,8% O xy= O xybutynin,F la= F lavoxate,C le= C lenbuterol,Prov= Propiverine,Pro = Propanth eline,Pl= Placebo,R C T = R andom C ontrolledTrial,N S = N otsignificant Overactive bladder 158 of 217 Final Report Update 4 Drug Effectiveness Review Project Evidence Table 4. A ntich olinergicoveractive bladdersyndrom e drugs com pared with non-drug th erapy N um ber A ge A uth or, Study Design screened/ G ender Y ear Setting eligible/enrolled Eth nicity Interventions (drug,regim en,duration) G oode R CT 486screened, M eanage67 O x y 2. Behavioraltherapy asdescribedinBurgio1998 of Burgio following the E thnicity 100% addedtoO x y patients 1998) R CT reportedin white G oode2002 O xy= O xybutynin,B eh = B eh avior,Pl= Placebo,EN S = ElectricalN erveStimulation Overactive bladder 159 of 217 Final Report Update 4 Drug Effectiveness Review Project Evidence Table 4. A ntich olinergicoveractive bladdersyndrom e drugs com pared with non-drug th erapy A uth or, O th erpopulationch aracteristics Y ear (diagnosis,etc) Eligibility criteria Exclusioncriteria G oode 48% m ix edty peincontinence Age> = 55y rs,am bulatory ,urgeincontinence> /= Continualleakage,postvoidresidual> 2002 Severity of urinary incontinence:54% severe, 2x /wkforatleast3m onths,urody nam ic evidenceof 200m l,uterineprolapsepasttheintroitus, 20% m ild bladderdy sfunction. Burgio2001 SeeG oode2002 SeeG oode2002 SeeG oode2002 Burgio Ty peof U rinary Incontinence:U rgeonly (%)= 49. SeeBurgio1998 forinitialeligibility O xy= O xybutynin,B eh = B eh avior,Pl= Placebo,EN S = ElectricalN erveStimulation Overactive bladder 160 of 217 Final Report Update 4 Drug Effectiveness Review Project Evidence Table 4. A ntich olinergicoveractive bladdersyndrom e drugs com pared with non-drug th erapy N um berwith drawn/ A uth or, lostto follow-up/ M eth od ofO utcom e A ssessm ent Y ear analyz ed and Tim ing ofA ssessm ent O utcom es G oode 92ex cludedfrom analy sis:28didnot Bladderdiary R eductioninVoiding frequency /24h:O x y -2. R esultsin reasonsnotreported) 9subscalesandaG lobalSeverity 155analy z ed Index ,50onany scaleisnorm al,63+ is"ex trem eenough tobeacase" Burgio 24withdrew/0losttof/u/190analy z ed Bladderdiaries,patientsatisfaction Changeinincontinenceepisodes: 1998 andoverallevaluationof perceived O x y 10. O x y ) Insubgroup of wom en(n= 131)with nocturia M eanreductioninnocturiafrom baseline: O x y :0. Behavioralto (ex tension analy z ed com binedtherapy 57. A ntich olinergicoveractive bladdersyndrom e drugs com pared with non-drug th erapy A uth or, A dverse effects assessed? W ith drawals due to Y ear H ow assessed adverse events C om m ents G oode N otreported N otreported N otenough datapresentedtofully evaluateresults. Burgio2001 Seeabove Seeabove Thisisasubgroup analy sisfrom theBurgiostudy ,of thosecom pleting psy chologicalanaly sis. N otreported 1998 D ry m outh O x y 97%,Beh 35%,Pl55% Inability tovoidO x y 22%,Beh 6%,Pl3% ConstipationO x y 39%,Beh 22%,Pl37% BlurredvisionO x y 15%,Beh 10%,Pl10% ConfusionO x y 8%,Beh 6%,Pl11% Burgio2000 N otreported N otreported Thisisasubgroup analy sisof patientsagreeableto (ex tension com binedtherapy postBurgio1998trial. A ntich olinergicoveractive bladdersyndrom e drugs com pared with non-drug th erapy N um ber A ge A uth or, Study Design screened/ G ender Y ear Setting eligible/enrolled Eth nicity Interventions (drug,regim en,duration) Soom ro R andom iz ed 43enrolled, M eanage O x y 2. Patientscontrolledam plitudeto Singlesite reported produceatickling sensation,at20Hz frequency andpulseof 0. Colom bo R CT 81screened, Age:O x y = 48, O x y 5m g threetim esdaily orbladdertraining x 6weeks 1995 Singlesite othersnot Beh= 49 U SA reported 100percentfem ale E thnicity not reported O xy= O xybutynin,B eh = B eh avior,Pl= Placebo,EN S = ElectricalN erveStimulation Overactive bladder 163 of 217 Final Report Update 4 Drug Effectiveness Review Project Evidence Table 4. A ntich olinergicoveractive bladdersyndrom e drugs com pared with non-drug th erapy A uth or, O th erpopulationch aracteristics Y ear (diagnosis,etc) Eligibility criteria Exclusioncriteria Soom ro M eanfunctionalcapacity 154 Patientswith ahistory of frequency ,urgency and N otR eported 2001 urgeincontinencewhohadnotbeenpreviously treatedatthedepartm ent,including som ewhohad previously receivedtreatm entfrom ageneral practitioneratleast6m onthspriortostudy enrollm ent. Colom bo D etrusorinstability :O x y = 14,Beh= 13; Patientsshowing detrusorinstability ,low- Stablebladderatcy stom etry ;neurologic 1995 L ow-com pliancebladder:O x y = 9,Beh= 8; com pliancebladderandsensory bladder disease;detrusorhy perreflex ia;agegreater Sensory bladder:O x y = 15,Beh= 16 than65y ears;coex isting genuinestress urinary incontinence;genitalprolapse; postvoidresidualvolum egreaterthan50m l; previousgy necologicalorurogy necological operation;prioruseof any drug forthe treatm entof urinary urgeincontinence; urethraldiverticula;fistulas;urinary tract neoplasia;bacterialorinterstitialcy stitis; bladderstones;andpreviouspelvic radiotherapy O xy= O xybutynin,B eh = B eh avior,Pl= Placebo,EN S = ElectricalN erveStimulation Overactive bladder 164 of 217 Final Report Update 4 Drug Effectiveness Review Project Evidence Table 4. A ntich olinergicoveractive bladdersyndrom e drugs com pared with non-drug th erapy N um berwith drawn/ A uth or, lostto follow-up/ M eth od ofO utcom e A ssessm ent Y ear analyz ed and Tim ing ofA ssessm ent O utcom es Soom ro N otR eported Voiding diary ,Bristolurinary R eductioninvoiding frequency /24h:O x y -2,E N S:-2 2001 sy m ptom questionnaireandQ uality Sy m ptom sby Bristolurinary sy m ptom questionnaire: of L ifequestionnaire significantchangesinscoreinboth groupsonfrequency ,and dissatisfactionwith spending restof lifewith currentsy m ptom s com paredtobaseline N odifferenceonleaking orhesitancy com paredtobaseline O x y only hadsignificantchangeinscoreforincom pleteem pty ing com paredtobaseline SF -36: N osignificantdifferencescom paredtobaseline Patientsfinding treatm enteffective: O x y 10,E N S 4 Colom bo 6withdrawn:O x y = 4dueto Clinicalcure:totaldisappearanceof Clinicalcure: 1995 anticholinergic adverseevents;Beh= 2 urgeincontinenceanddidnotrequire D etrusorinstability group:O x y = 93%,Beh= 62% consentwithdrawals protectivepadsorfurthertherapies L ow-com pliancebladdergroup O x y = 67%,Beh= 75% Sensory bladdergroup:O x y = 60%,Beh= 81% O xy= O xybutynin,B eh = B eh avior,Pl= Placebo,EN S = ElectricalN erveStimulation Overactive bladder 165 of 217 Final Report Update 4 Drug Effectiveness Review Project Evidence Table 4. A ntich olinergicoveractive bladdersyndrom e drugs com pared with non-drug th erapy A uth or, A dverse effects assessed? W ith drawals due to Y ear H ow assessed adverse events C om m ents Soom ro Post-treatm entsideeffectsquestionnaire(at N otreported 2001 6wks) D ry m outh O x y 87%,E N S 6% BlurredvisionO x y 53%,E N S 6% D ry skinO x y 30%,E N S 28% SkinirritationO x y N A,E N S 11% D ifficulty using m achineE N S 13% Colom bo U nclear. O x y = 4(3duetodry 1995 O x y :D ry m outh= 15;constipation= 6; m outh;1dueto N ausea= 5;D iz z iness= 2;D ecreaseinvisual glaucom a) acuity = 1;Tachy cardia= 1; Beh = nonereported Beh = nonereported O xy= O xybutynin,B eh = B eh avior,Pl= Placebo,EN S = ElectricalN erveStimulation Overactive bladder 166 of 217 Final Report Update 4 Drug Effectiveness Review Project Evidence Table 5. New overactive bladder syndrome drugs compared with placebo Mean Change in Number Author of Incontinence Year Dose Mean Change in Number of Micturitions/24h Episodes/24h OAB drug Placebo OAB drug Placebo (n) (n) (n) (n) Rentzhog TOL ↓20% (not given) Not reported ↓46% Not 1998 2mg BID (not given) reported Jacquetin TOL ↓1. Q uality assessm entofplacebo-controlled trials Internalvalidity A uth or, R andom iz ation A llocationconcealm ent G roups sim ilarat Eligibility criteria O utcom e assessors C are Y ear adequate? Zinner,2006 Yes N R Yes Yes "D ouble-blind"m ethods Yes N R Staskin,2007 Yes Yes Yes Yes Yes Yes Hill,2006 Yes Yes Yes Yes Yes Yes D m ochowski, Yes Yes Yes Yes "D ouble-blind"m ethods Yes 2008 N R Chapple,2007 Yes Yes Yes Yes Yes Yes Overactive bladder 171 of 217 Final Report Update 4 Drug Effectiveness Review Project Evidence Table 6. Q uality assessm entofplacebo-controlled trials A uth or, Patientm asked? R eporting ofattrition, A ttrition: Intention-to-treat(ITT) Q uality F unding Y ear crossovers,adh erence,differential/h igh analysis R ating and contam ination Zinner,2006 Yes Attritiony es(15% N o Yes F air N ovartis overall) Pharm aAG CrossoverN R Protocolviolationswere reported Staskin,2007 Yes Attritiony es(11. Q uality assessm entofplacebo-controlled trials R udy ,2006 N otreported N otreported Yes Yes Yes Yes R ackley ,2006 N otreported N otreported Yes Yes Yes Yes Overactive bladder 173 of 217 Final Report Update 4 Drug Effectiveness Review Project Evidence Table 6. Q uality assessm entofplacebo-controlled trials R udy ,2006 Yes Attrition-Yes,Crossover- Yes,L O CF F air Indevus N o,Adherence-N R , N o/N o Pharm aceut Contam ination-N R icalsInc N on com pleters: Trospium ‐7. A ssessm entofabstracts forpublicationbias M icturitions U rge incontinence A uth or Interventions m eanch ange episodes m eanch ange Y ear (Drug,dose,sam ple siz e) (tim e period) (tim e period) H ead-to-h ead VanK errebroeck A:Tolterodine2m g BID (n=120) A:-2. A ssessm entofabstracts forpublicationbias M icturitions U rge incontinence A uth or Interventions m eanch ange episodes m eanch ange Y ear (Drug,dose,sam ple siz e) (tim e period) (tim e period) M oore A:Tolterodine1m g BID A:-1.
Lenalidomide maintenance was found to benefit therapy or a second transplantation to deepen response minocycline 50mg online. Two all patient subclassifications generic minocycline 50mg with mastercard, including cytogenetic risk and remis- ongoing trials will help to update and refine the evidence for sion status, in the IFM 05-02 study. The third lenalidomide up-front versus delayed autologous HSCT and help to determine maintenance study of 402 patients has been reported in abstract which patient populations should proceed to autologous HSCT and form and, in addition to comparing maintenance versus no mainte- 33 which patient populations can defer transplantation. The Dana nance, compared chemotherapy versus tandem autologous HSCT. Farber Cancer Institute trial in conjunction with the IFM will The median PFS was 37. The IFM group has completed accrual from diagnosis was 76% for maintenance and 68% for no mainte- nance (P. The 81% for maintenance and 72% for no maintenance, respectively U. There was no difference in SPM rates between the This will provide an indirect comparison of the effect of 1-year maintenance and no maintenance arms. The European Myeloma Network (EMN) will examine the role of chemotherapy versus single versus tandem autologous HSCT. In all Table 557 lists current treatment recommendations for MM. Risk arms, the maintenance of lenalidomide 3 weeks per month is given stratification at diagnosis will help with selecting treatment and until progression. Other strategies are ongoing to incorporate vaccina- autologous HSCT. It tion against MM antigens, along with immunomodulatory agents remains to be determined whether RVD consolidation will improve such as IMiDs or the anti-PD-1 antibody. The incorporation of new outcome after single autologous HSCT. The BMT-CTN 0702 is a agents into the treatment of MM patients should lead to further phase 3 study examining a single autologous HSCT followed by a prolongation of response and long-term control of the disease. All 3 arms are followed by 3 years of lenalidomide Disclosures maintenance therapy. The and has received honoraria from Celgene and Janssen. Off-label drug use: thalidomide, after chemotherapy and single or tandem transplantation. McCarthy, Roswell Park Cancer Institute, BMT Program, are recommended as the primary agents to be considered for Department of Medicine, Buffalo, NY 14263; Phone: 716-845-4074; long-term maintenance. Bortezomib may be considered for 2 years Fax: 716-845-3272; e-mail: philip. There has been no demonstrated increase in SPMs with 1. Lenalidomide maintenance until disease cation of monoclonal gammopathies, multiple myeloma and progression was shown to improve PFS and OS in the CALGB related disorders: a report of the International Myeloma Work- 100104 study, especially in patients receiving lenalidomide-based ing Group. How I treat while not showing an OS benefit at this time, showed a PFS benefit multiple myeloma in younger patients. High-risk cytogenetics maintenance and that for progression and death is higher in patients and persistent minimal residual disease by multiparameter flow not on maintenance therapy in the CALGB 100104 study. The risk cytometry predict unsustained complete response after autolo- of SPM development should be evaluated within the context of the gous stem cell transplantation in multiple myeloma. European perspec- dations for very-high-risk disease. Therefore, for very-high-risk tive on multiple myeloma treatment strategies: update follow- patients with plasma cell leukemia; t(14;16), del(17p), del(1p), and ing recent congresses. Bergsagel PL, Mateos MV, Gutierrez NC, Rajkumar SV, San bortezomib and lenalidomide maintenance therapy can be considered. Improving overall survival and overcoming adverse prognosis in the treatment of cytogenetically high-risk multiple Summary and future directions myeloma. The treatment of the MM patient has improved over the past 10 6. Consensus years, with median PFS approaching 4 years after autologous recommendations for risk stratification in multiple myeloma: HSCT. New agents based on MM cell metabolic pathways of report of the International Myeloma Workshop Consensus growth and cell development and antibodies that have activity with Panel 2. Combining fluores- treatment of relapsed and refractory disease. Such agents include novel PIs such as marizomib and the oral 8. The molecular PIs ixazomib and oprozomib, the recently Food and Drug Adminis- characterization and clinical management of multiple myeloma tration (FDA)–approved PI carfilzomib, the recently FDA-approved in the post-genome era.

In a good-quality case-control study order minocycline 50 mg overnight delivery, children (ages 7 to 19) who had died from “sudden unexplained death” during the years of 1985 to 1996 were identified from state vital statistics 276 from each of the 50 United States order 50 mg minocycline with amex. A control group was selected from children who died from motor vehicle traffic accidents, with 1:1 matching resulting in 564 in each group. The exposure was defined as stimulant use immediately prior to death, based on survey of parents. Sensitivity analyses altering the way exposure was identified or removing children also taking a tricyclic antidepressant did not meaningfully alter the results. Recall bias is the greatest risk in this study, as the time since the child’s death to the survey of the parent was longer in the sudden death group (13 years) compared with the motor vehicle death group (10 years). In a fair-quality retrospective cohort database study, the risk of stroke or transient ischemic attack was compared in adults taking initiating atomoxetine or stimulant therapy for ADHD/ADD (N=42 993). Using propensity score matching and only validated cases of stroke or transient ischemic attack, no statistically significant difference was found between the drugs for either outcome (relative risks, 1. Overall the numbers of cases were small, limiting the ability to determine statistically significant differences if they were to exist. Blood pressure, pulse, electrocardiographic changes Lisdexamfetamine. Three trials of lisdexamfetamine 30, 50, or 70 mg daily compared with placebo reported various intermediate outcome measures of cardiovascular function. Small increases in blood pressure and heart rate were seen in all 3 studies, with none being deemed clinically important. A small, open-label, uncontrolled 11-month study of lisdexamfetamine in 272 children did not find any cases of “clinically relevant” changes in blood pressure or 277 electrocardiographic parameters. A 4-week, placebo-controlled trial of 314 adolescents reported increases in heart rate of 2 to 3 beats per minute with lisdexamfetamine (1 beat per 184 minute increase with placebo). Two patients, both taking 70 mg daily doses of lisdexamfetamine, discontinued drug after 1 week due to increased QTc intervals. Additionally, a 4-week randomized, placebo-controlled trial of 30 mg, 50 mg, and Attention deficit hyperactivity disorder 85 of 200 Final Update 4 Report Drug Effectiveness Review Project 70 mg of lisdexamfetamine in 420 adults (54% male, mean age was 35. Although statistical analysis of between-group differences was not reported, for lisdexamfetamine 30 mg, 50 mg, 70 mg, and placebo, respectively, rates for blood pressure increase were 1%, 3%, 4% and 0%, for increased heart rate were 1%, 3%, 3%, and 0%, for palpitations were 2%, 1%, 3%, and 0%, and for tachycardia were 1%, 3%, 0%, and 278 0%. An open-extension of a trial of methylphenidate OROS reported small changes in blood pressure (3. During this time, 33% discontinued treatment, but only 1 withdrew due to systolic blood pressure >130 mmHg. ANOVA analyses showed no relationship to dose or age and no tolerance development over time was found, but those children with the lowest blood pressure at baseline had the greatest increases. The final report from this 2-year study found no 280 additional withdrawals due to cardiovascular adverse events. In a 7-week study of 226 adults (56% male, mean age of 39 years), similar proportions of participants in the methylphenidate OROS and placebo groups, respectively, had systolic blood pressure greater than 140 mmHg at any post baseline visit (8% compared with 6%), but greater proportions of participant in the methylphenidate OROS group had diastolic blood pressure greater than 90 mm Hg (10% compared with 3%; P not reported) and a pulse rate of greater than 227 100 bpm (7% compared with 2%; P not reported). Four open-label extension studies of mixed amphetamine salts 281, 282 283 XR, 1 each in children, adolescents, and adults examined the cardiovascular effects over 284 periods of 6 to 24 months. In each of these studies the subjects were populations of patients who were highly selected and were described as being healthy other than the diagnosis of ADHD. The studies in children and adolescents also included a short-term placebo-controlled phase. While no statistically significant differences compared with placebo in any electrocardiogram measure were found in children in the short-term trial, 2% (11/568) had diastolic blood pressure >90 mmHg, and 9% (50/568) had a systolic blood pressure >130 mmHg at some point during follow-up. In a shorter duration open-label study, 2968 children were given mixed amphetamine salts XR for a 282 period of up to 15 weeks. The absolute numbers of patients with cardiovascular adverse events were not clearly reported. Nine patients had treatment emergent cardiovascular adverse events that were moderate or serious in intensity, 5 of which were deemed probably related to mixed amphetamine salts XR. Thirteen of 79 adolescent patients (16%) experienced adverse events during a 4-week study of mixed amphetamine salts XR compared with placebo that included cardiovascular 283 symptoms such as syncope, tachycardia, and electrocardiogram abnormality. Of these, 2 were withdrawn from study drug, 1 with palpitations and 1 with severe migraine and syncope. During 6-month follow-up there were no serious cardiovascular adverse events reported, although 4% (6/138) reported adverse events with cardiovascular symptoms, however none withdrew due to these adverse events. In a 2-year extension study in adults with ADHD, two-thirds discontinued Attention deficit hyperactivity disorder 86 of 200 Final Update 4 Report Drug Effectiveness Review Project 284 the study prior to completing 2 years, 22% because of adverse events. Statistically significant, but not considered clinically meaningful, increases in systolic blood pressure and diastolic blood pressure were seen at various points throughout the study (mean increase in systolic blood pressure, 2.
Side effects: the usual PI side effects buy minocycline 50mg cheap, with (moderate) gastrointestinal complaints and dyslipidemia cheap 50mg minocycline otc. The dyslipidemia may not be as pronounced as with other PIs. Interactions, warnings: caution for sulfonamide allergy. Since darunavir is metab- olized by the cytochrome P450 system, some interactions have to be taken into account. Lopinavir and saquinavir lower the plasma levels of darunavir and should be avoided. John’s wort, astemizole, cisapride, midazolam, ergotamine derivatives, rifampicin, phenytoin, and carbamazepine. Use atorvastatin instead of pravastatin at the lowest dose (10 mg). Dosage adjustments may be required with efavirenz (decreased darunavir levels and increased efavirenz levels), rifabutin (dose should be reduced to 150 mg every two days), calcium antagonists (increased levels), methadone (reduced levels). Maximum doses of PDE5 inhibitors when combined with darunavir, 10 mg Cialis in 72 hours; 2. Comments: Well-tolerated and broadly applicable HIV protease inhibitor that has considerable activity against PI-resistant viruses. Different dosages as well as interactions have to be taken into account. For detailed information see page: 93 Dasabuvir Manufacturer: AbbVie. Indications and trade name: In combination with ombitasvir + paritaprevir + riton- avir (Viekirax) for adult patients with chronic hepatitis C, genotype 1. No dose adjustment is required even for patients with severe renal impairment. Side effects: the most common side effects are fatigue and nausea. Combination 12 weeks with Viekirax in genotype 1b (cirrhosis plus ribavirin), with Viekirax plus ribavirin in genotype 1a (cirrhosis duration 24 weeks). Numerous drug interactions especially with ritonavir which is provided as part of Viekirax. Do not combine with efavirenz, nevirapine, Drug Profiles 689 etravirine, beware of rilpivirine (higher levels, QT prolongation). HIV PIs should be given only unboosted, the fixed-dose lopinavir/r and cobicistat containing regimens are contraindicated. Comments: non-nucleoside NS5B polymerase inhibitor for hepatitis C GT1. Efficacy data in HIV-coinfected patients is limited. Numerous interactions with ART have to be considered as dasabuvir is usually given with the ritonavir-boosted HCV PI pari- taprevir (see Vikierax). For detailed information see page: 458 Daunorubicin (liposomal) Manufacturer: Gilead Sciences (Galen Limited), Fresenius Indications and trade name: AIDS-associated Kaposi sarcoma with <200 CD4 T cells/µl and severe mucocutaneous or visceral involvement. Side effects: during infusion: back pain, flushing (up to 14%). Symptoms usually occur during the first minutes and resolve when the infusion is slowed or stopped. Interactions, warnings: liposomal doxorubicin is contraindicated in decompen- sated cardiomyopathy, severe myelosuppression (neutrophils <1,000/µl, platelets <50,000/µl). Cardiological examination is important (ECG, echocardiography: left ventricular ejection fraction, LVEF) before initiation of treatment and at periodic intervals during treatment. Comments: compared to pegylated liposomal doxorubicin, treatment with liposo- mal daunoribicin yields to slightly lower remission rates of KS. However, as capac- ity constraints for Caelyx were seen in the past years, DaunoXome represents an alternative for KS treatment. Indications and trade name: HIV infection, in combination with other antiretro- viral agents. Pancreatitis, even after longer periods of treatment. Rarely lactic acidosis, especially in combina- tion with d4T and ribavirin. The following drugs should be used with caution: d4T, etham- butol, cisplatin, disulfiram, INH, vincristine (peripheral neuropathy). Concurrent dosing with tenofovir should be avoided because it increases the AUC of ddI by 44%. Treatment with indinavir, dapsone, ketoconazole, itraconazole, or tetracyclines should be given 2 hours before or after ddI. Initially, monthly monitoring of amylase, blood count, transaminases and bilirubin.